The increasing digitalization of the pharmaceutical and medical device industry has created novel cybersecurity challenges, particularly with the rapid advancement of artificial intelligence (AI) technologies. This article examines the dual nature of AI as both a potential threat vector and a powerful defensive tool.
The move to digital transformation represents a true paradigm shift in manufacturing, enabling organizations to leverage advanced technologies such as the Industrial Internet of Things (IIoT), cloud computing, and artificial intelligence (AI) to ensure compliance and secure a competitive advantage. This article presents a working definition of digital transformation, the components involved in...
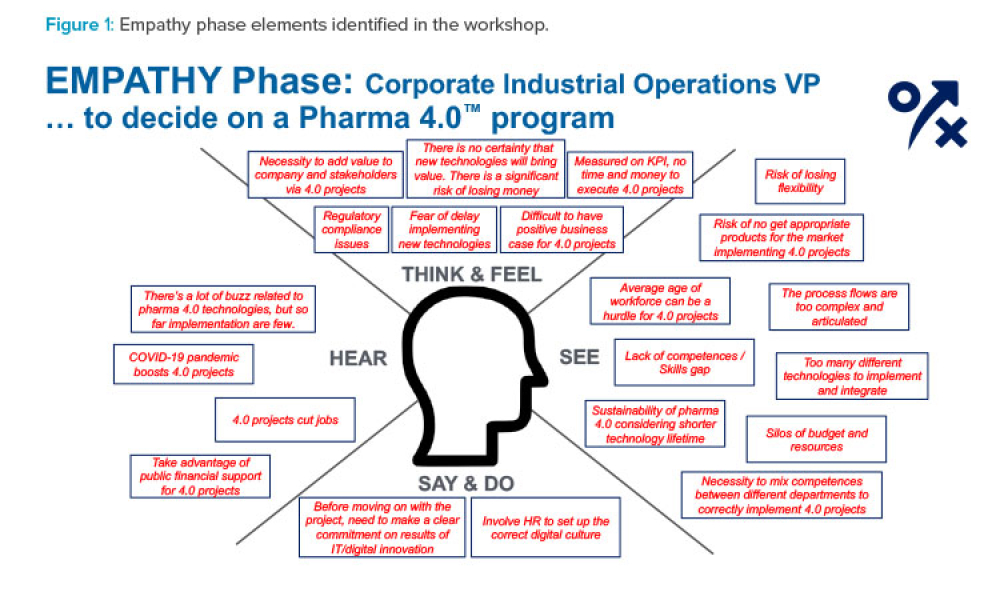
Pharmaceutical companies can use digital maturity assessments to address the challenges of upgrading brownfield facilities and implementing digital transformation improvements. Enabled by stakeholder workshops, these assessments can rapidly produce concrete plans and priorities to guide a facility’s development over the next three to five years—delivering business value and laying the...
Pharma 4.0™ is a reference framework tailored to the pharmaceutical industry, guiding its digital transformation. Although many of today’s processes generate sufficient data to enable advanced use cases, structured guidance for transformation is often lacking. To address this, the ISPE Pharma 4.0™ Subcommittee on Process Data Maps and Critical Thinking has introduced an approach to help...
The often-overlooked function of chemistry, manufacturing, and controls (CMC) holds tremendous potential to reshape the landscape of drug development. By embracing innovation and rethinking traditional approaches, CMC has the rare opportunity to drive transformative changes that could significantly accelerate the journey from initial concept to a fully realized therapy. This offers the...
The ISPE Baseline® Guide Vol 8: Pharma 4.0™ was published in December 2023 to help accelerate the adoption of digital transformation. In quoting the opening narration of the original 1960s Star Trek television series, Christian Wölbeling, Founder and Chair of the ISPE Pharma 4.0™ Community of Practice, likes to say, “To boldly go where no one has gone before.”
With the help of Digital Twins, companies can achieve greater certainty and precision in making informed decisions at various stages of the product lifecycle, driven by a deep understanding of underlying Critical Business Parameters (CBPs).
Informed consent aims to provide clinical trial participants with transparent education about the trial’s objectives, potential risks and benefits, and procedural requirements. As clinical trial designs evolve to include decentralized and adaptive elements, the informed consent process becomes increasingly complex. In response, this article explores the potential application of AI-powered...
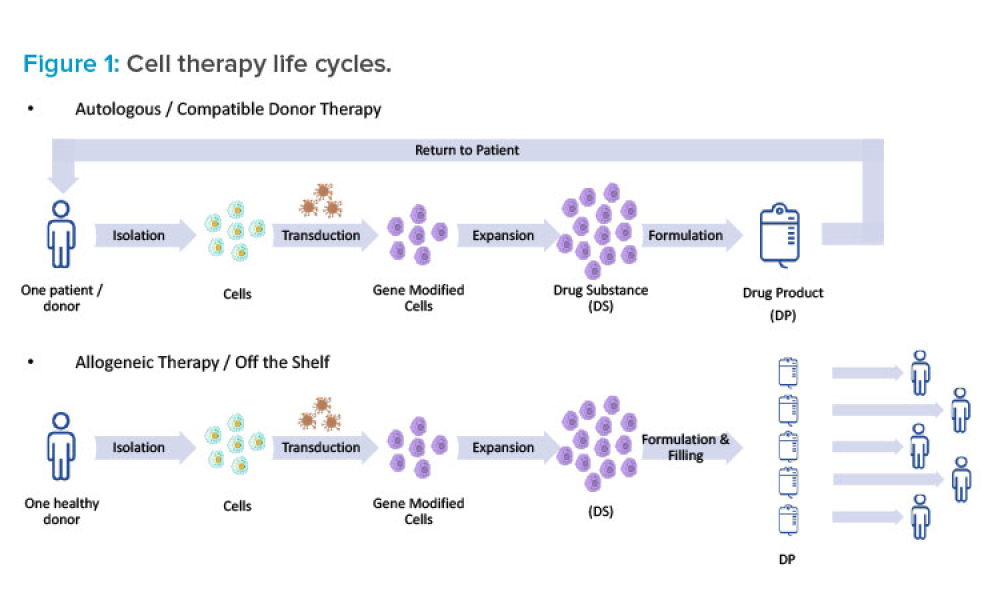
Cell therapies, especially autologous chimeric antigen receptor T cell (CAR T cell) treatments, are transforming personalized medicine, bringing new hope to patients with conditions once thought untreatable. However, the manufacturing processes for these therapies remain predominantly manual, presenting significant challenges in scalability, consistency, and making these treatments more widely...
Advanced therapy medicinal products (ATMPs), which include cell and gene therapy (C>) products, frequently require handling steps between quality control release and patient administration. These steps take place directly at the point of care and are especially critical for C>s with limited shelf life after preparation.
Advanced therapy medicinal products (ATMPs) have the potential to treat life-threatening, incurable conditions. But access to these therapies remains challenging due to the nature of current ATMP manufacturing models. This article explores solutions, focusing on standardized processing and shared knowledge as gateways to automated, robotic manufacturing and decentralized production.
For patients who depend on personalized medicine, turnaround time matters. However, moving quickly is difficult for cell therapy companies because designing personalized therapies presents unique challenges unknown in traditional biotechnology. In this article, we’ll examine five strategies to help cell therapy companies develop resilience against these challenges, positioning themselves to...
Advanced therapy medicinal products (ATMPs) are transformative therapeutics that are realizing increasing gains in market approvals, yet are expensive products to produce. To enable a broader application of these medicinal products in the marketplace, the cost of goods (COGs) sold should be addressed early in development with a focus on reduction of cost to the patient.
A growing segment of the advanced therapy medicinal product (ATMP) landscape, which includes gene therapies and cell-based treatments, relies heavily on viral vectors for efficient gene delivery. The increasing demand for these therapies requires a robust, scalable, and cost-effective manufacturing solution.
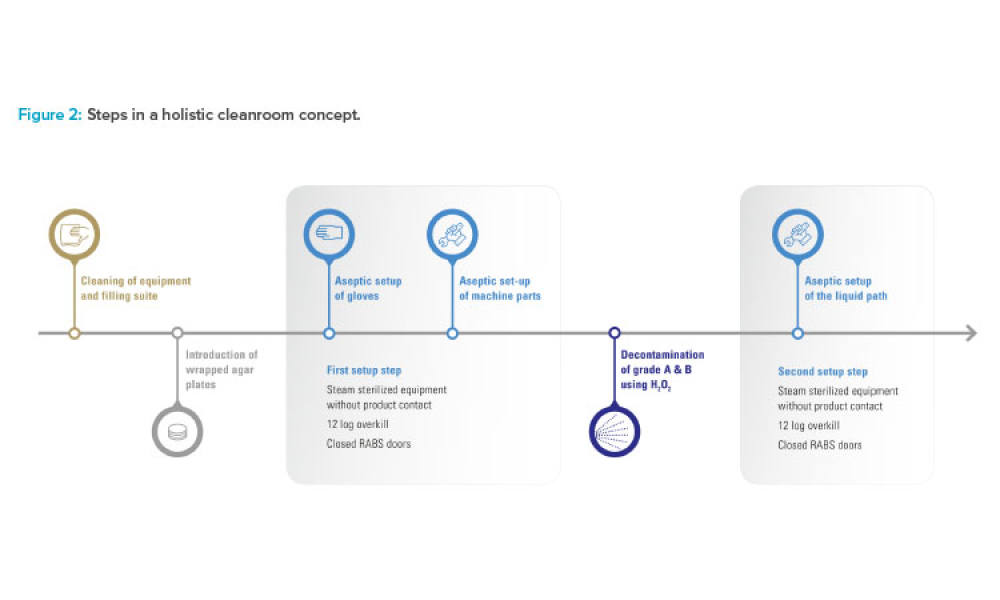
Controlling contamination in environments where biological medicinal products are handled is of paramount importance to ensure the safety of personnel, sterility of drug products, and protection of the surrounding environment. The application of vaporized hydrogen peroxide (vH2O2) has emerged as a promising method for postproduction decontamination due to its ability to...
For companies focused on producing lifesaving treatments, the positive effects of employee health, well-being, and satisfaction can be easily overlooked, but those positive effects are real. An investment in people results in better research, testing, and manufacturing processes, which leads to more efficient delivery of therapies and treatment to patients worldwide.
Organizations must continually evolve and adapt in order to grow, sustain, and stay competitive. No organization survives for a long period of time if it does not change with the times. The pace of change is accelerating, and the scale of disruptive market forces is growing by the day.
The landscape of clinical trials has been transformed in a post-pandemic world. In July, ISPE released the second edition of ISPE GAMP® Good Practice Guide: Validation and Compliance of Computerized GCP Systems and Data – Good eClinical Practice. It addresses managing complexities associated with decentralized clinical trials, the benefits and challenges of using open-source software, and...
This article describes a practical and pragmatic approach to the management of computerized system life cycle and information technology (IT) process records. The objective is to effectively achieve and maintain compliant GxP-regulated systems that are fit for intended use, and to support patient safety, product quality, and data integrity.
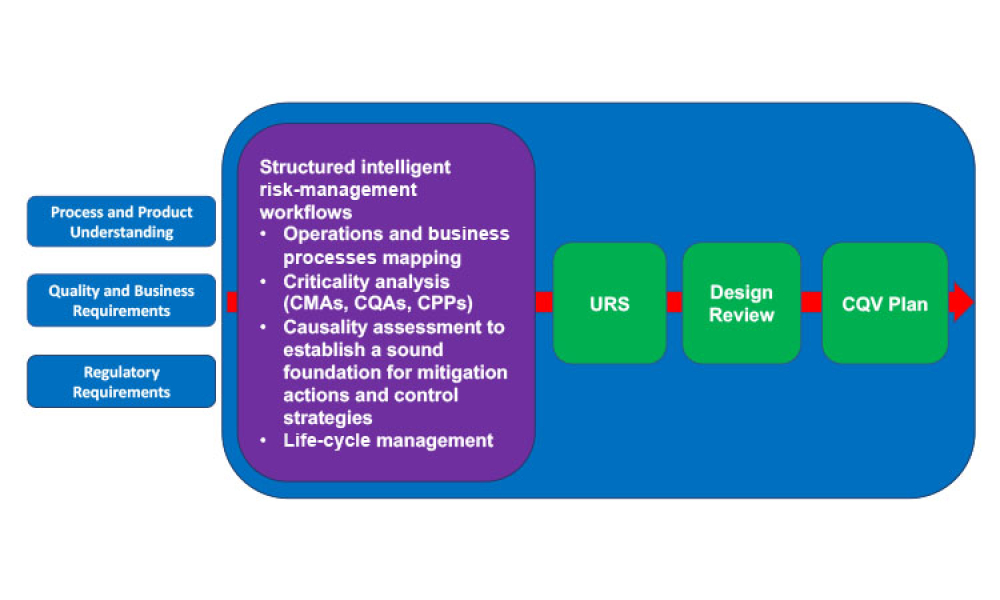
Risk assessment is essential, however, risk assessment is of limited value if it is not conducted by a team with the necessary process, product, and functional understanding. Conducting risk assessments prematurely may lead to invalid assessment of overall risks. Conducting risk assessments too late will limit the opportunity to address design flaws and effectively test processes and...
This special anniversary article addresses the history and milestones that define the GAMP Community of Practice (CoP). In celebration of the 25th anniversary of the creation of GAMP Americas, we reflect on the vital role GAMP Americas has played in that journey. We commemorate key accomplishments of its members, share recent activities, and look ahead to the future of GAMP Americas.
This article presents a comprehensive analysis of plastic waste generation in the biopharmaceutical industry, focusing on using single-use technologies (SUT) in bioprocessing for monoclonal antibody (mAb) production. It aims to inform sustainable practices within the biopharmaceutical industry and to encourage the development of more sustainable disposable technologies.
This article describes the numerous activities in the commercial quality control (QC) network that aim to replace in vivo assays with alternative methods in the course of production and release.1
Reducing the pharmaceutical industry’s carbon footprint has become a management responsibility. This article introduces some of the key points, actual methods, and practical examples of our implementation to reduce carbon emissions from pharmaceutical manufacturing facilities in Southeast Asia.
Pharmaceutical and biotechnology companies employ platform analytical procedures in the development stages of their synthetic and biological drug products and are beginning to leverage them for commercial products. This shift is supported by the acceptance of platform procedures in the recently adopted ICH Q2(R2) and ICH Q14. Six case studies are shared in this article to highlight how...
The pharmaceutical industry faces considerable challenges throughout the development, manufacturing, and supply of medicines, largely due to the intricate and divergent global regulatory landscape. The adoption of structured data standards and utilization of cloud-based platforms offer immense potential to overcome these challenges by facilitating faster and more efficient global...
This article offers an overview of the benefits of Pharmaceutical Inspection Co-operation Scheme (PIC/S) membership for regulatory authorities and industry. It also highlights Latin American regulators’ current perspectives on PIC/S membership to increase awareness and encourage open dialogue about harmonization, recognition agreements, and potential increase for export facilitation, all which...
The pharmaceutical industry stands at the precipice of a revolution as emerging digital technologies provide new opportunities to boost productivity through continuous process improvements. The Pharma 4.0™ framework, an adaptation of the broader Industry 4.0 movement, aims to transform how drugs are produced and delivered.
With the approval of the first gene edited therapeutic in 2023, production of gene edited therapies is accelerating, introducing tough decisions for manufacturing development. Gene editing therapy production is complex, often involving multi-modality manufacturing operations in one facility to produce a single therapeutic. This article considers whether retrofitting an aging monoclonal...
Implementing advanced automation technologies is a strategic move that can amplify the positive outcomes of environmental, social, and governance (ESG) initiatives. By leveraging ESG initiatives, pharmaceutical companies can enhance their competitive edge and contribute positively to global sustainability efforts.
The explosive growth of advanced therapy medicinal products (ATMPs), particularly cellular therapeutics, has driven steady investment in facilities capable of manufacturing these therapeutics at scale . Meanwhile, the industry is collectively moving to adapt to European Union Annex 1 standards, which places a more stringent emphasis on contamination control.
Cell and gene therapy (C>) products represent a significant step forward in patient treatment and often offer unique patient benefits. However, product developers face significant hurdles within the regulatory landscape. The tools laid out in the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) Q12 guideline: “Technical and...
The US Food and Drug Administration (FDA) advocates for the integration of quality by design (QbD) principles throughout the pharmaceutical product development landscape, aiming to elevate both process understanding and product quality. Key challenges to the process control strategy include navigating time- and resource-intensive processes. One solution is digital shadow technology which, when...
In the dynamic and highly regulated world of biopharmaceutical manufacturing, maintaining and ensuring quality is a critical success factor. An effective quality risk management (QRM) system is a key component in the overall quality management infrastructure of biopharmaceutical organizations. It offers a structured, scientific, and risk-based approach to decision-making, addressing potential...
Advanced therapy medicinal products (ATMPs) and cell and gene therapies (C>s) represent a promising medical product class that employs gene therapy, cell therapy, or tissue engineering to address various diseases and injuries. One critical aspect of ATMP and C> manufacturing is using cell culture media. With thousands of ATMPs and C>s in clinical trial phases, the role of...
Antibody engineering has transformed the development of therapeutic antibodies, enabling the creation of specific and effective treatments for a range of diseases. These antibody-based therapeutics are advancing in clinical development at a rapid rate and are being approved in record numbers. Currently, more than 100 monoclonal antibodies (mAbs) have been approved for the treatment of various...
Although traditional tank farm systems have long been the cornerstone of buffer preparation, they face challenges that have grown with the expansion of processing scale in the industry.
The commercialization of personalized medicine has ushered in demand for a new type of facility—personalized medicine facilities—which can produce thousands of small-scale batches per year. There are currently only a handful of these sites, but many more are in various stages of design and construction. Designing these personalized medicine facilities presents new challenges, and a different...
With the Chinese government initiating drug regulatory reform in 2015 and China joining the International Council for Harmonisation (ICH) in 2017, a significant number of measures have been implemented by the government. The aim is to make fundamental changes to China’s drug regulatory administration system so it can facilitate pharmaceutical development and better meet patient needs in the...
Digital display labels (DDLs) offer an alternative solution to eliminate manual relabeling in the clinical supply chain, optimizing label content updates through a simple, system-controlled approach while providing new, uncharted opportunities. With increased efficiency in making regulatory-compliant changes and enhanced flexibility in the clinical supply chain, DDL technology has the...
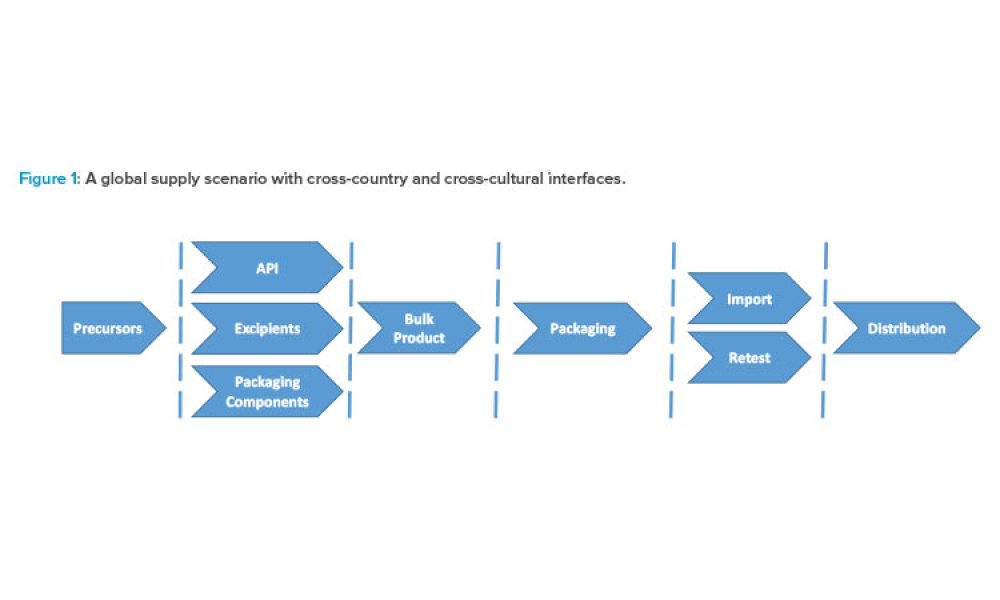
The Asia Pacific region (APAC), like any large territory, encompasses a blend of well-established and early-stage economies, diverse healthcare systems, and differences in language, culture, politics, and technology adoption. APAC’s size and complexity has created new challenges and opportunities for the pharmaceutical industry as nations work together to meet the manufacturing needs for...
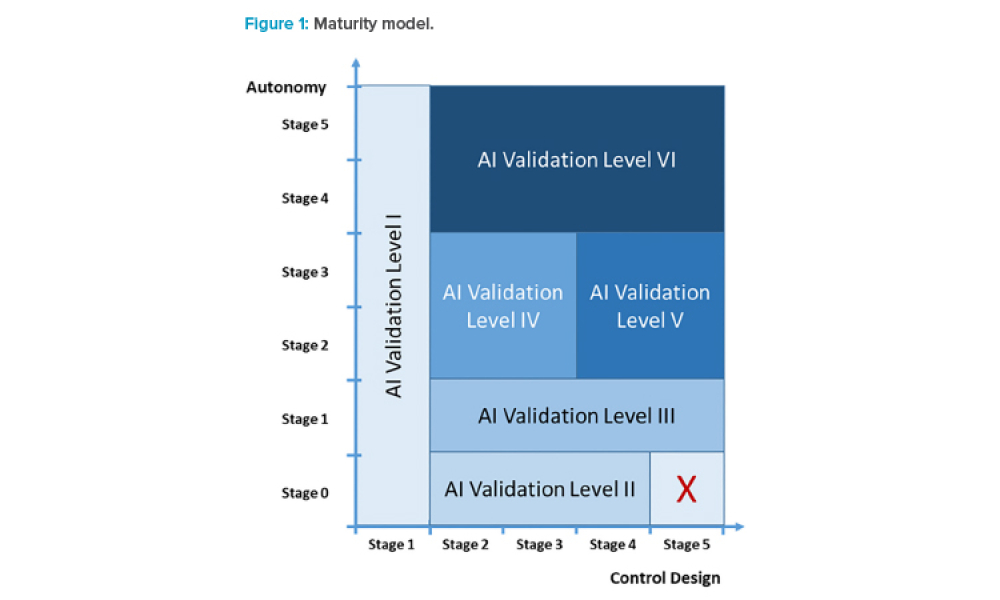
Stakeholders across industries are becoming accustomed to using information technology (IT) systems, applications, and business solutions that feature artificial intelligence (AI) and machine learning (ML). Even though some of these uses show phenomenal performance, thorough risk management is required to ensure quality and regulatory compliance are met within the life sciences industry. By...
Due to the growing digitalization of the industry, we are highly dependent on information technology (IT) systems and data. The basic ability to execute our pharmaceutical business and decision-making processes relies on the permanent availability of these IT systems and data to ensure compliance and efficiency of our business operations. But numerous factors—including criminal activities,...
IT infrastructure has traditionally been provisioned using a combination of scripts and manual processes. This manual approach was slow and introduced the risk of human error, resulting in inconsistency between environments or even leaving the infrastructure in an unqualified state. In this article, we investigate some fundamental advantages of using Infrastructure as Code (IaC) for...
This article provides a brief introduction into the standards and regulations for medical devices. It compares the ISPE GAMP® 5 Guide: A Risk-Based Approach to Compliant GxP Computerized Systems (Second Edition) and applicable ISPE GAMP Good Practice Guides against the relevant regulations and standards for the development of software for medical devices and demonstrates GAMP® 5 Second...
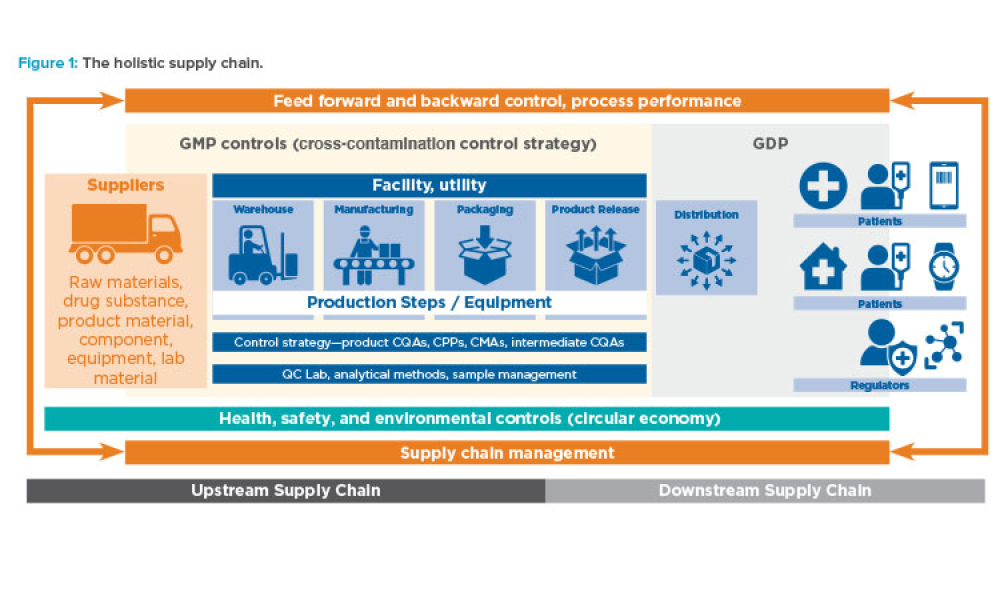
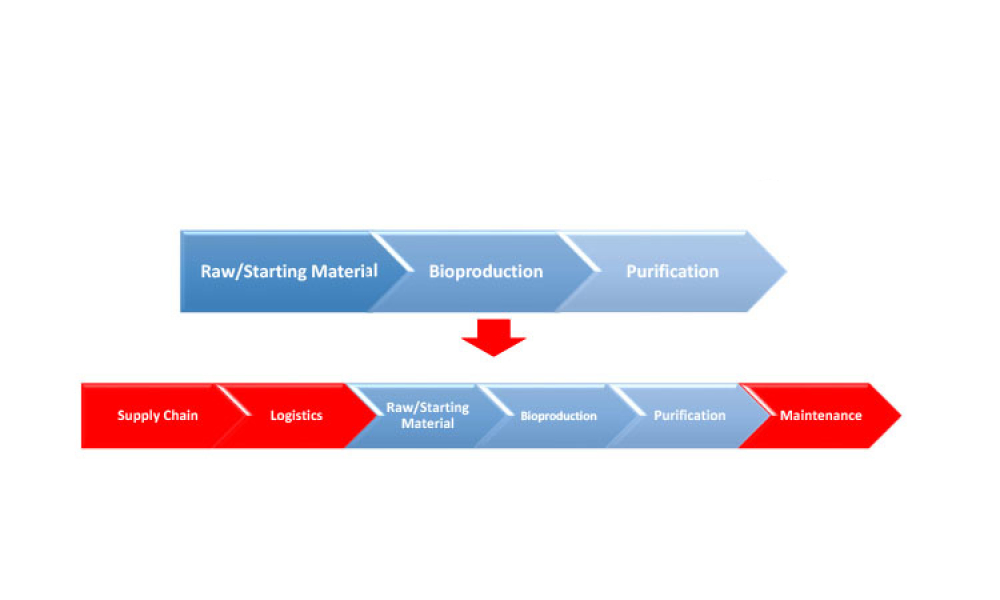
Advanced therapy medicinal products (ATMPs) are one of the most promising developments in the pharmaceutical and biotech industries in recent decades. Although there is a great promise to treat and even cure many diseases with these products, there are also unique challenges, especially with their supply chains.
Facility design decisions made early in conceptual design can have a significant impact on the cost of goods sold (COGS) in the manufacture of autologous and allogeneic cell therapy products. Understanding the impact of a COGS analysis is an important aspect of the early-phase design process.
Live biotherapeutic products (LBPs) have the potential to treat a wide range of ailments. However, these living microorganisms are difficult to produce due to evolving government regulations and limited GMP manufacturing experience. New facility designs and more specific process guidance could help overcome these challenges. This article explores the nuances of facility design and regulatory...
Cell and gene therapy (C>) products comprise a rapidly growing field of innovative medicines that hold the promise to treat and, in some cases, cure diseases that are otherwise untreatable. In this article, we provide points to consider when evaluating the comparability of C> when changes are made in their manufacturing processes.
There is much that large-scale commercial stem cell therapy processes can adopt from the existing bioprocessing industry. This article addresses some of the unique challenges posed by large-scale stem cell and stem cell–derived product manufacturing processes, and what should be considered while designing a manufacturing facility.
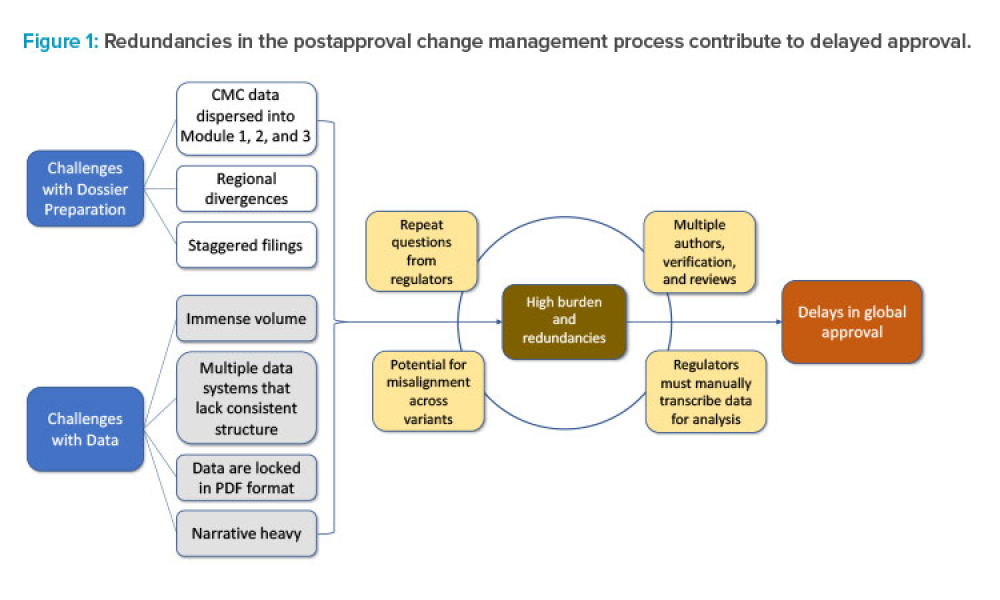
As the demand for accelerated access to medicines expands globally, the pharmaceutical industry is increasingly submitting regulatory applications in multiple countries simultaneously. As a result, Boards of Health (BoHs) are challenged with approving these applications in an accelerated timeframe and accommodating the submission of postapproval chemistry, manufacturing, and controls (CMC)...
The new European Commission GMP Annex 1 “Manufacture of Sterile Medicinal Products” and the equivalent Annex 2 from the World Health Organization (WHO) triggered a discussion in ISPE’s Germany/Austria/Switzerland (D/A/CH) Aseptic Processing Community of Practice (CoP) Steering Committee about where to qualify air speed: “at working position” versus “at working level.” This article provides...
The biopharmaceutical industry must develop and implement innovative ways of working to be effective and efficient in the current healthcare ecosystem, in which high-quality medicines, adaptability, and assurance of supply are of critical importance. There are regulatory strategies and technologies emerging to address these challenges, but further progress must be made to fully harness the...
This article describes how ISPE GAMP® 5: A Risk-Based Approach to Compliant GxP Computerized Systems (Second Edition) and related GAMP Good Practice Guides can be effectively applied to help meet the requirements of the proposed European Union (EU) artificial intelligence (AI) regulation for qualifying GxP-regulated systems employing AI and machine learning (ML).
ISPE has launched an important new initiative, “Enabling Global Pharma Innovation: Delivering for Patients,” in support of the aspirations of many regulatory agencies globally to promote introduction of innovative pharmaceutical manufacturing.
Shortages of essential medicines around the world have been an ongoing concern for patients, caregivers, and regulators and have been exacerbated by the COVID-19 pandemic. Many regulators have instituted requirements for reporting potential or actual drug shortages.
Understanding and managing risks to continuous manufacturing (CM) technology is central to any decision to greenlight CM in a production-ready environment. Applying a systemwide risk management (SRM) approach to manufacturing is essential to ensuring manufacturing projects are vetted in a comprehensive and consistent manner.
Pharmaceutical continuous manufacturing (CM) is recognized as a key process intensification technology, with investment expected to rise in the coming years and the focus shifting toward biologics. This article provides a review on the current state of CM implementation, offers insights into life cycle management and regulatory aspects, and explains how a data- and knowledge-centric approach...
In the interest of understanding the current state of continuous manufacturing for biologics and to facilitate the path toward adoption of these promising technologies, the United States Pharmacopeia (USP) and BioPhorum jointly sponsored a hybrid workshop. This article summarizes trends from the workshop and ponders next steps.
Continuous manufacturing (CM) challenged regulators’ expectations and regulatory frameworks. This article discusses how US regulators addressed the regulatory hurdles related to CM to broaden its adoption through engagement, regulatory science, guidance, and international harmonization.
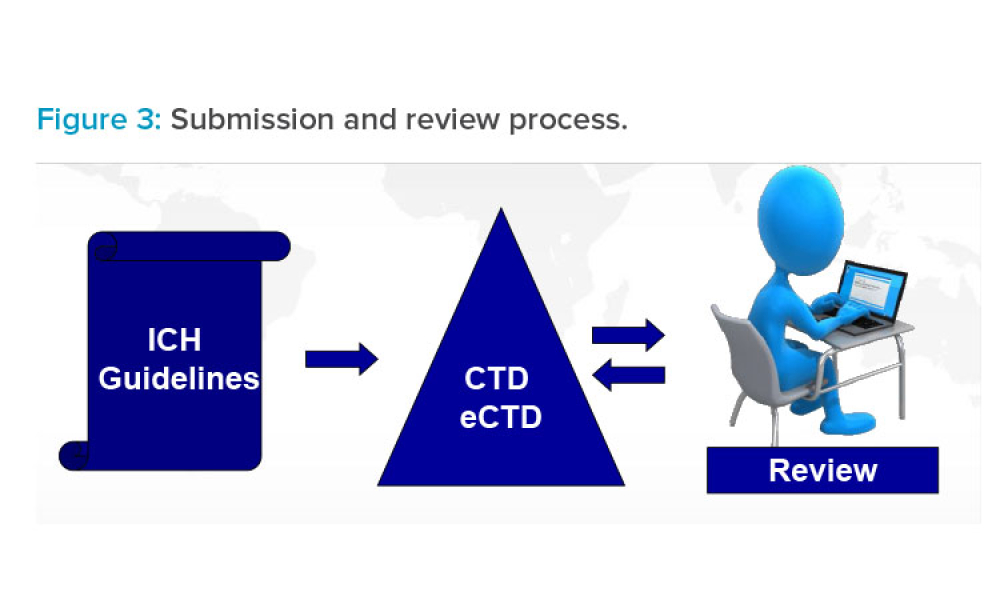
When working with the common technical dossier (CTD), the structure of Module 2 “follows the scope and outline of the Body of Data in Module 3,”
Funded by the European Commission from 2019, the Smart Pharmaceutical Manufacturing Project (SPuMoNI)
The global pandemic has demonstrated that now, more than ever, we need to work toward a global solution and prioritize the harmonization of technical requirements. Positive improvements have been observed in the acceptance and implementation of international standards by various regulatory agencies in Latin America. This article offers an overview of the chemistry, manufacturing, and controls...
The success of the biopharmaceutical industry and the expansion of manufacturing facilities, of both existing companies and newcomers, has put a strain on the number of temporary and permanent skilled workers needed to fill many positions in the Triangle.
North Carolina’s Research Triangle is the largest of its kind in the US. Thanks to years of effort from industry, pharmaceutical professionals, and education institutions, it is synonymous with pharmaceutical and biotechnology excellence.
While financial investment in novel therapies provides patients with new treatment options and improved quality of care, the pharmaceutical industry also recognizes its responsibility to transition toward more sustainable development, manufacturing, and stewardship of medicines throughout their life cycle.
To enable changes across the pharmaceutical industry, sustainability should be included alongside quality, efficacy, and safety when assessing medicines. This article reviews two case studies that cover sustainable pack types and extension of shelf life. With the drive to manage unmet medical need through acceleration of drug development programs, postapproval sustainability variations will...
The imperative for global action to tackle climate change is clear and the pharmaceutical industry has a key role to play. Governments have entered into international commitments to reduce climate impact (carbon emissions) and protect nature (water, land, air, and biodiversity) with policy frameworks established to facilitate and drive progress against agreed targets.
The expected FDA approval for a Treprostinil dry powder inhaler revealed a need for the manufacturer to expand its warehousing and logistics capabilities to support its growing operations. The company’s senior leadership wanted to ensure this expansion came with as minimal an impact on the environment as possible, so a key priority was to provide a net zero energy facility. With a vision for...
As the pharmaceutical industry faces ever-changing global challenges and market forces, it must review and revise product design to ensure that quality products remain available in the marketplace while moving toward zero pollution for air, water, and soil. This article provides an introduction on how quality products can integrate sustainability by design.
Recent advances in artificial intelligence (AI) have led to its widespread industrial adoption, with machine learning (ML) algorithms demonstrating advances in performance in a wide range of tasks. However, this comes with an ever-increasing complexity of the algorithms used, rendering such systems more difficult to explain.
ISPE’s GAMP® 5: A Risk-Based Approach to Compliant GxP Computerized Systems (Second Edition) (GAMP® 5 Guide, 2nd Edition) maintains the principles and framework of the first edition and updates their application in the modern world, including the increased importance of service providers, evolving approaches to software development, and expanded use of software tools and...
In this article, potential Pharma 4.0™ technological solutions that can enhance continuous process verification (CPV) 4.0 are discussed. The necessary paradigm shift will allow companies to predict deviations more accurately, perform root cause...
This article explores life-cycle activities for machine learning (ML) within regulated life sciences. It positions and contextualizes the life cycle and management of the machine learning subsystem or components within a wider system life cycle. It also gives general descriptions and guidance illustrated by a case study demonstrating a machine learning application to medical image recognition,...
Many organizations are evaluating how advanced therapy medicinal products (ATMPs) and other traditional modalities may be combined within the same facility or within a newly constructed agnostic building. This article outlines a broad framework to evaluate different types of modalities that may be accommodated concurrently in a new or existing facility and then uses a case study to explain how...
Cell and gene therapies (C>) have unique needs in manufacturing suites that differ from those for classic product biopharmaceuticals. Facilities must be created with flexibility in mind, able to run multiple products and production types to remain viable.
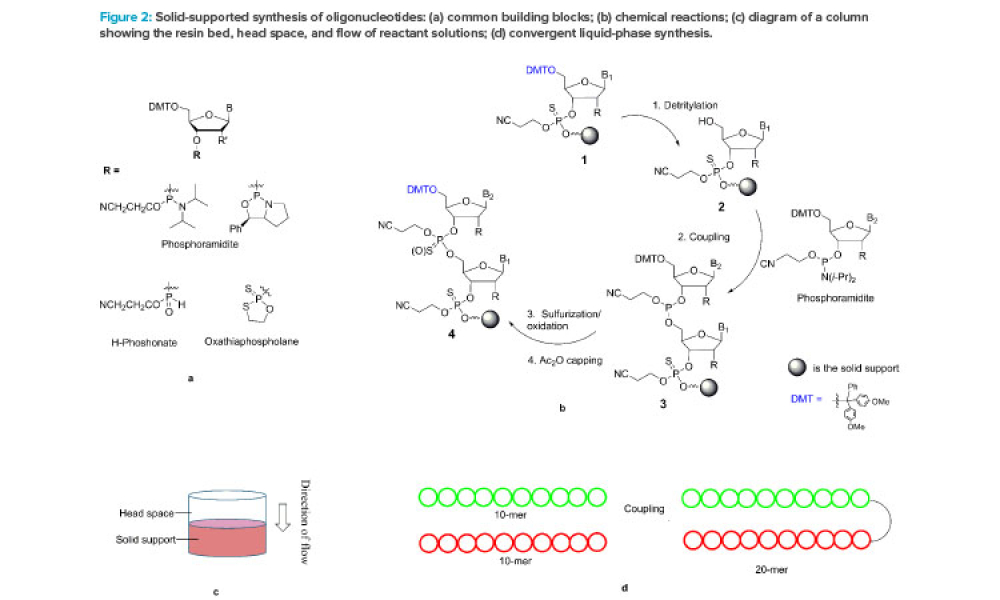
Resounding clinical successes and maturation of extensive therapeutic pipelines have catapulted oligonucleotides from a fringe modality to therapeutic relevance in just a few short years. Oligonucleotides are a cornerstone of a burgeoning class of drugs classified as nucleic acid therapeutics. These therapies interact with DNA and RNA targets rather than traditional protein therapeutic...
Digital health is transforming the health care landscape through new technologies and platforms in patient care management, conducting of clinical trials, patient data collection, and the diagnosis and treatment of disease. Emerging digital health technologies (DHTs) may improve the quality of life for patients with chronic and debilitating diseases and provide novel health care solutions for...
A reliable supply of raw materials is critical to maintain a robust supply chain to serve patients globally. With shortages, regulatory complexity is compounded due to differences in submission and data requirements from various regulatory agencies. Therefore, there is an increasing need to implement a harmonized regulatory infrastructure that is both flexible and predictable to provide more...
Postapproval change management of pharmaceuticals is an essential part of life-cycle management but is associated with regulatory challenges. Incorporating concepts and tools from the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) Q12 guideline, combined with structured content and data management (SCDM) and a cloud-based data exchange...
The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) guideline on Registration of Pharmaceuticals for Human Use (M4)
Although data and knowledge are both stand-alone disciplines that need to be systematically managed, they also must have a connection. Understanding the relationship between data and knowledge management processes and how people are leveraging advances like Pharma 4.0™ combined with these processes enables quality data transition to knowledge that can help pharmaceutical companies. The authors...
As the pharmaceutical industry continues to grow and evolve, a significant contributor to innovation and evolution is mergers and acquisitions (M&A). M&A can enable academic researchers and small companies to fund and commercialize innovative products. In addition, M&A can help larger organizations secure new and complementary technology and products. In the pharmaceutical...
As the industry experiences significant changes to the way we do business, knowledge capture and sharing are more important now than ever before. The maturing digitalization of the biopharma industry’s business and processes is creating an increasingly data- and information-rich environment that requires more effective mechanisms for sharing data and information. The Knowledge Management team...
On 26 January 2022, representatives of the author team for the ISPE Good Practice Guide: Knowledge Management in the Pharmaceutical Industry held a webinar to provide an...
ISPE held an Expert Xchange on 18 January 2022 entitled “Risk-Based Decision Making: Advancing the Integration of Quality Risk Management (QRM) and Knowledge Management (KM).” The session included presentations and interactive exercises that generated new and useful insights into the current effectiveness of the knowledge that flows into QRM and how a knowledge map can be used to diagnose...
In a recent advanced therapy medicinal products (ATMPs) innovator survey, two-thirds of respondents reported that they are developing multiple drug platforms.
The life cycle approach to process validation stresses the need for continued monitoring of process performance to ensure that the manufacturing process remains stable and predictable, i.e., in a state of control. This life cycle stage is known as continued process verification (CPV) or ongoing process verification (OPV).
Two sessions at the 2022 ISPE Facilities of the Future Conference in early February captured varied views of emerging technologies in the pharmaceutical industry, and the industry’s work to embrace...
What do recipients of ISPE’s prestigious Facilities of the Year Award (FOYA) know that has helped their projects succeed? What are the lessons learned from achievements in facilities development, including forward-looking projects that encompass and inspire changes in the industry? Pharmaceutical Engineering® spoke with nine FOYA winners from recent years about the lessons they learned and...
Realizing the promise of any novel viral vector therapeutic depends on the innovator’s ability to constantly meet evolving program requirements set in the product’s preclinical; clinical; chemistry, manufacturing, and controls (CMC); and market strategies. A key enabler to success is establishing a robust yet nimble viral vector manufacturing platform that delivers high-quality product on time...
As adoption of cloud technology continues to increase across the life sciences industry, so too does the need to establish a standardized and pragmatic approach for ensuring the quality of software applications used in support of GxP data and associated processes. This article focuses on the application level and the growing use of software as a service (SaaS) within the life sciences...
Artificial intelligence (AI) has become one of the supporting pillars for digitalization in many areas of the business world. The pharmaceutical industry and its GxP-regulated areas also want to use AI in a beneficial way. Several pharmaceutical companies are currently running digital pilots, but only a small fraction follows a systematic approach for the
With the publication of recent guidance, specifically the US FDA Quality Systems Approach to Pharmaceutical cGMP Regulations
This article aims to refresh information on open-source software (OSS) within regulated computerized systems that was first discussed in an article in May-June 2010 Pharmaceutical Engineering®. The adoption of OSS advanced since then, and the article explores the importance of recognizing when an organization is relying on OSS and the benefits and risks this brings from a GAMP® 5...
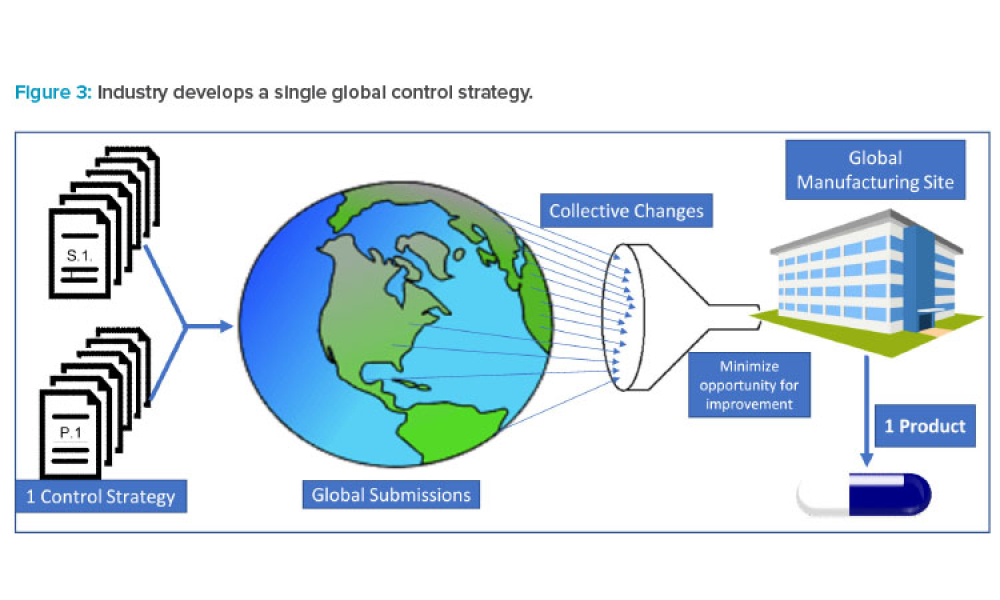
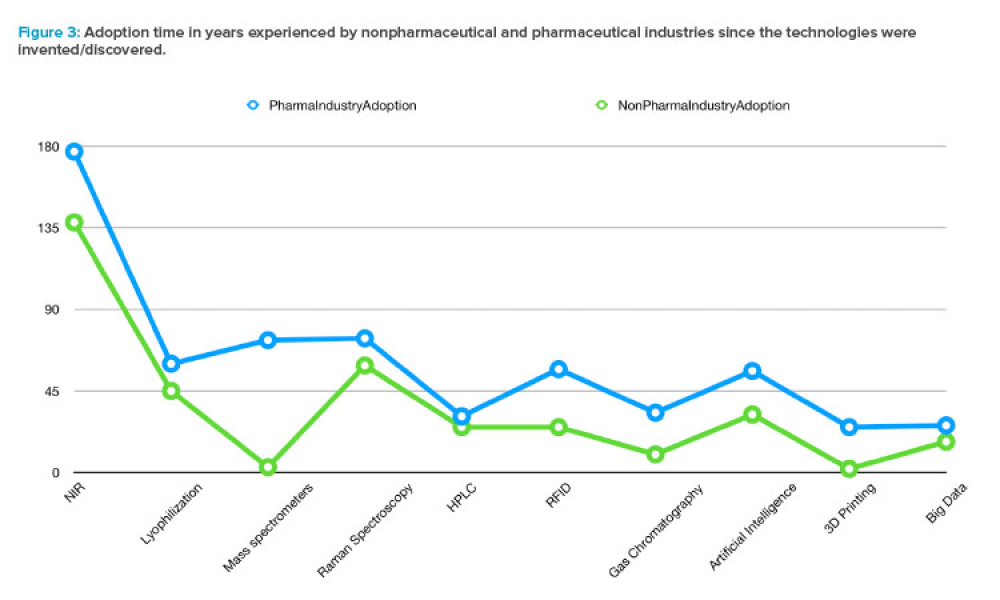
During the past decade, industry has experienced a proliferation of regulatory divergence regarding the interpretation and implementation of International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) guidelines (and control strategies) across geographic regions. This article shares data that highlight instances where well-established ICH...
With the rise of new technologies and predictive analytics capable of handling the huge amounts of data within and across existing information systems, Industry 4.0 has been thriving in many sectors, such as industrial automation, financial technology, retail, and semiconductors. But the health sector in general,
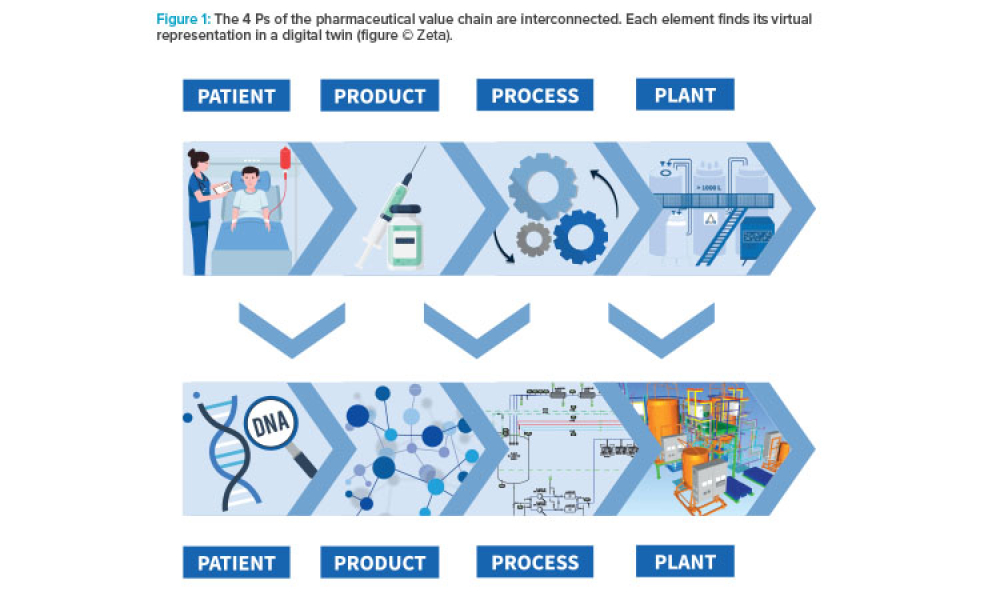
Developing comprehensive digital solutions is crucial for the entire value creation process for pharmaceuticals. A holistic view of the interrelations of product, production process, and plant is becoming increasingly significant. In this context, the application of model-based technologies provides support in drug development, process scale-up, and manufacturing. Furthermore, it accelerates...
Emerging Leaders has grown from an initiative for interactions among early-career professionals entering ISPE into much more: a training ground, a networking organization, and a new foundation for the future of ISPE and the industry. This article looks at the history of the group, its purpose, current and future initiatives, and a name change that better reflects the path of its members.
Industry 4.0 applications in biopharma involve the complete spectrum of data science throughout the entire product life cycle of many disparate entity types. Tools such as digitalization, modern data science, and the industrial internet of things (IIoT) exist now, and examples from other industries such as Siri and Alexa, face identification, and self-driving cars can guide their...
Historically, cell therapies are used to treat patients with cancer after relapse from other approved treatment modalities, or if no approved treatment is available. However, the introduction of allogeneic cell therapies has created exciting opportunities to broaden access to cell-based treatments. With advancements in manufacturing, developers are becoming increasingly interested in...
The process of bringing new drugs and products to market requires creativity, thinking outside the box, and the courage to fail numerous times before making a single discovery. This rings especially true now, as the industry faces the COVID-19 pandemic and doubts about vaccines and therapies getting to market in record time to improve human health. This article presents a deep dive into gene...
With so many options for personalizing our lives, is the personalization of medicine far behind? With all the data available, how can the industry bring personalized medicine to patients? This article explores what is currently available and where the pharmaceutical industry can move forward to better serve patient needs.
This article revisits the concept of phased engineering, procurement, and construction (EPC) and updates it with risk-based considerations specifically regarding the commissioning, qualification, and validation (CQV) of general life-cycle principles for pharma and biotech projects. Enhancing the relationship between phases of a project, advanced planning, and more formal management of...
The ISPE Global Pharmaceutical Regulatory Summit, held virtually on 28 April 2021, brought together 11 regulators from different parts of the world to discuss how their...
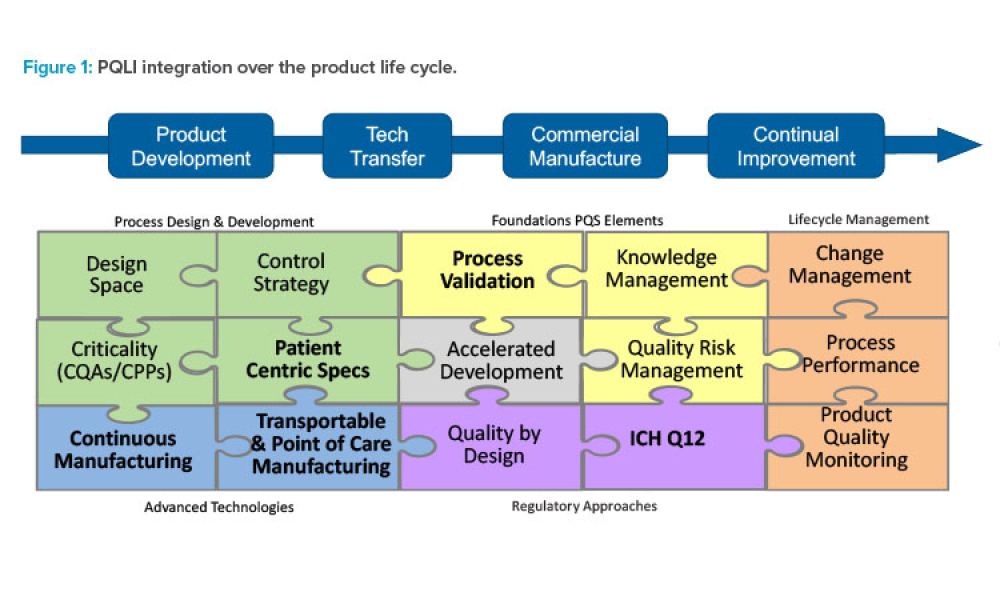
A unique aspect of the pharmaceutical industry is the pairing of innovation and regulation. For nearly two decades, ISPE’s Product Quality Lifecycle Initiative (PQLI®) has worked at the nexus of...
In its 30-year history, the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) has covered a wide range of topics to generate quality, safety, efficacy, and multidisciplinary harmonized guidelines. As science advances, issued guidelines are being updated and new guidelines are proposed in a pipeline of potential future activity. This...
ISPE’s Regulatory Affairs function plays a vital role in the Society, which is to build effective relationships with regulators and agencies globally and ensure all members have access to the latest regulatory developments and...
Management of global postapproval chemistry, manufacturing, and controls (CMC) changes is a growing challenge for industry with many issues. ICH Q12 (Technical and Regulatory Considerations for Pharmaceutical Product Lifecycle Management) is a transformative document shaping global regulatory postapproval submissions that will help alleviate some of these issues.
Continuous manufacturing (CM) offers one way the pharmaceutical industry can accelerate development of the drug product control strategy to ensure a robust and reliable supply of medicine to the clinic and/or market. This article explores the promise of continuous manufacturing in enhancing accelerated development, as evidenced by experience in solid oral products.
Application of continuous manufacturing (CM) in the pharmaceutical industry is gaining momentum. Most of the current experience is based on oral solid dosage (OSD) projects but in the future continuous manufacturing should not be limited to these dosage forms. In this article, the regulatory acceptability of continuous manufacturing to produce pharmaceuticals is demonstrated in different...
The ISPE OSD Community of Practice Continuous Manufacturing Subcommittee is planning a Good Practice Guide to capture information developed over several years by the team to establish equipment requirements, identify opportunities for harmonization and flexible integration, and suggest where current equipment may be enhanced to work with continuous manufacturing platforms of the future.
Cell and gene therapies are complex. As more therapies come to market in the hope of bringing advanced treatments and cures to rare, orphan, and difficult-to-treat diseases, designing quality standards for these personalized medicines is equally as complex.
Continuous manufacturing has attracted significant interest over the past decade for small molecules formulated as drug products. The case for adopting continuous manufacturing platforms for manufacturing biologics (i.e., large proteins or biologic products such as vaccines) would, in principle, be even more justified for both quality and business gains. This article briefly reviews continuous...
This second of a two-part series explores digital transformation and digitalization in the biopharmaceutical industry with information about how data science enables digitalization along the product life cycle. (Part 1 was published in the March-April 2021 issue of Pharmaceutical Engineering.
This article provides a beginner’s overview of how organizations can achieve a state of preparedness (readiness) for inspections, with a specific focus on IT systems.
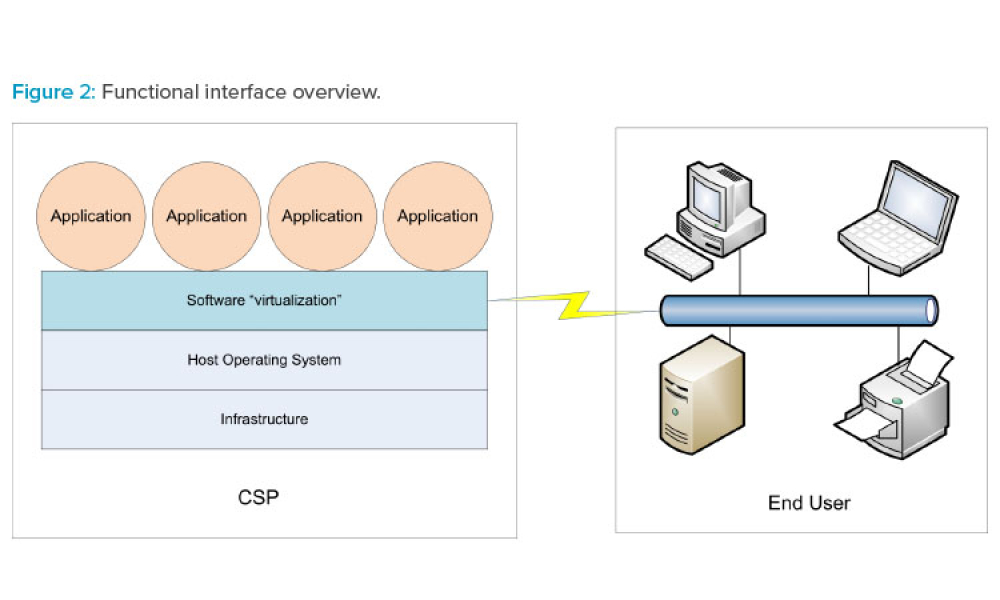
Cloud computing can be described as networked access and utilization of configurable computing resources such as data and information storage, processing capabilities, applications, and other services on computerized systems provided and/or maintained by a remote organization. As life sciences companies consider the advantages and costs of utilizing cloud services, they first need to invest...
In 2021, the ISPE GAMP® Community of Practice (CoP) is celebrating 30 years of promoting industry good practice for computerized systems and encouraging technical innovation and progress, while protecting patient...
Real-world evidence (RWE) is clinical evidence regarding the usage and potential benefits or risks of a medical product derived from analysis of real-world data (RWD) relating to patient health status and the healthcare delivery.
This article focuses on pragmatic quality- and risk-based approaches to IT infrastructure. It covers recommendations made by a US FDA/industry team linked to the US FDA Center for Devices and Radiological Health (CDRH) Case for Quality initiative
The ISPE Pharma 4.0™ Special Interest Group (SIG) launched in 2015 to provide a road map for new challenges of digitalization, Industry 4.0, and the smart factory. The Special Interest Group addresses how pharmaceutical industry stakeholders, including regulatory authorities, can achieve benefits from
The fourth Industrial Revolution (also known as Industry 4.0) is the era of smart machines, storage systems, and production plants that can autonomously exchange information, trigger actions, and control operations free of any human intervention. To ensure future success in the delivery of therapeutic medicines to patients, it is imperative that the pharmaceutical industry move deeper into the...
Digital transformation and digitalization are on the agenda for all organizations in the biopharmaceutical industry. But what are the main enablers of intelligent manufacturing? We hypothesize that data science–derived manufacturing process and product understanding is the main driver of digitalization in the bioprocessing industry for biologics manufacturing. In this article, the first of a...
During the ISPE Pharma 4.0™ Virtual Conference, the Management Communication working group of the ISPE Pharma 4.0™ Special Interest Group (SIG) held a workshop to support ISPE members in pitching,...
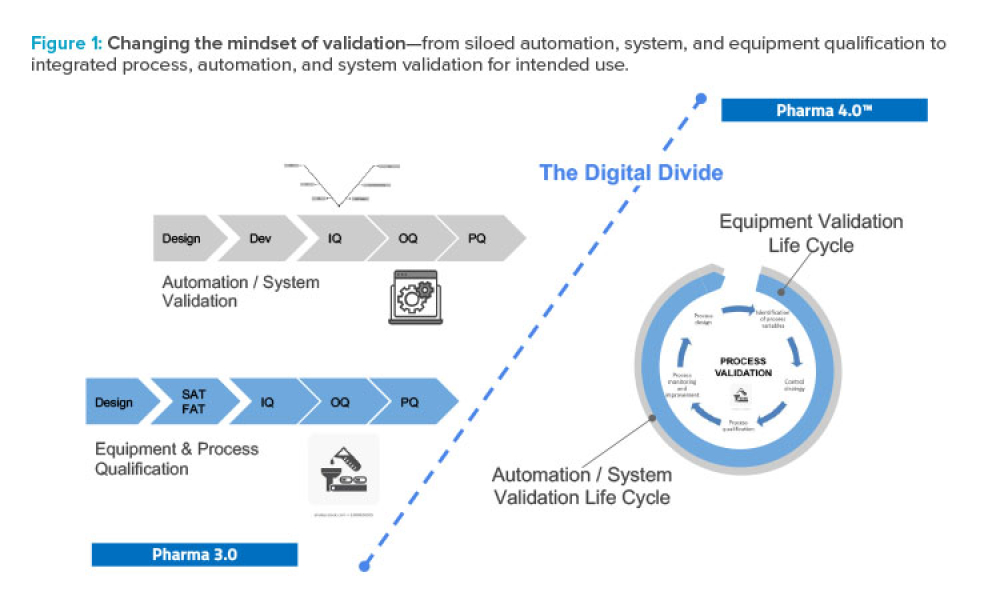
Across every industry today, digitalization is driving the use and value of data to disrupt traditional business models and ways of working. In pharmaceuticals, the promises of Industry 4.0 are expected, and needed, to finally modernize the legacy approaches that have evolved since the 1970s. Validation is an obvious target for digital disruption because of the inefficient, document-heavy...
Through this difficult time of the COVID-19 pandemic, ISPE has remained active. At the 2020 ISPE Pharma 4.0™ Virtual Conference, 17–18 November, 174 attendees gathered online to discuss and learn about...
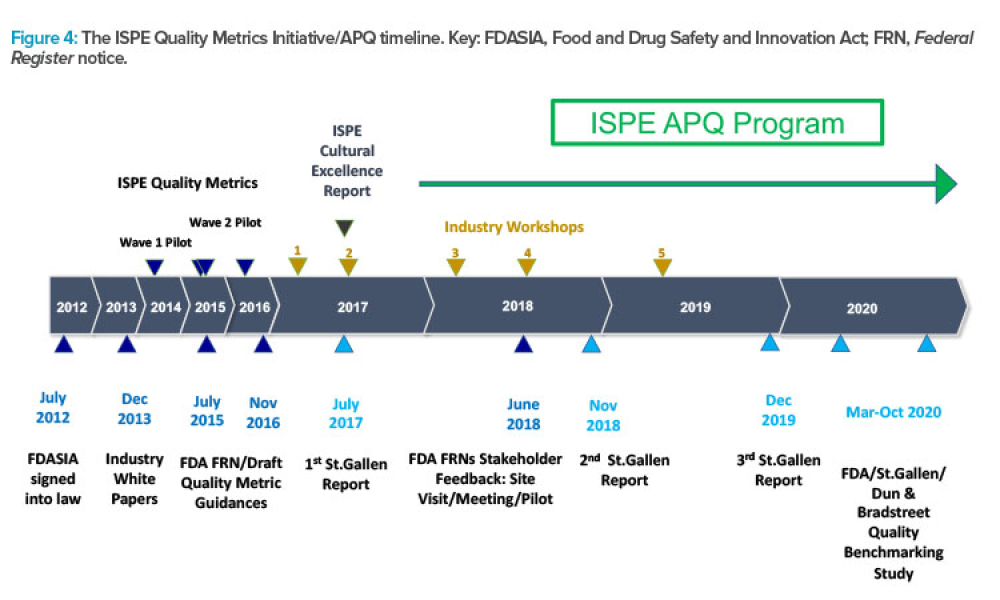
ISPE has announced the launch of its Advancing Pharmaceutical Quality (APQ) Program with the publication of the ISPE APQ Guide: Corrective Action and Preventive Action (CAPA)...
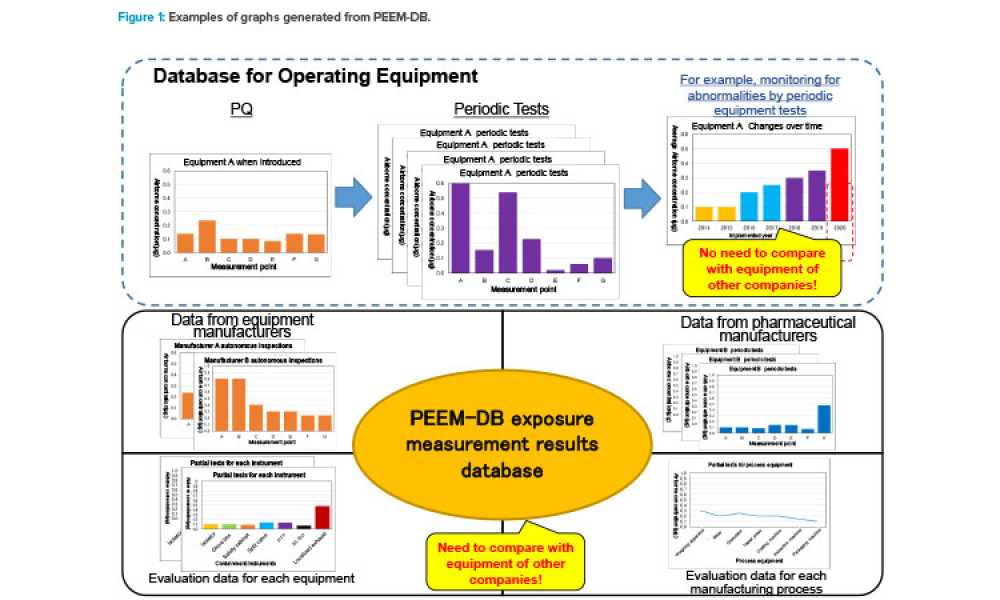
This article describes the Pharmaceutical Equipment Exposure Measurement Database (PEEM-DB), which was launched in July 2019 by the ISPE Japan Affiliate for its members. PEEM-DB is offered as a tool for rationally advancing optimal containment equipment settings by collecting exposure measurement results for...
Personnel management is the most challenging variable in maintaining current Good Manufacturing Practice (cGMP) across the life cycle of drug manufacture, safety, and supply. A standard operating procedure (SOP) outlines agreed-upon instructions for personnel training and instructions for maintaining systems, machines, documents, and records in a qualified state to produce safe products. This...
Drug developers know that the odds of anyone compound demonstrating safety and efficacy for a disease and its affected populations are low. How can drug developers improve these odds and increase the efficiency and effectiveness of drug development? One useful tool is model-informed drug development (MIDD), which uses computer models to inform the design of clinical trials or to run...
Women in Pharma® is a place where women and men—especially those new to the industry—can access a network of mentors, role models, and educational resources to support their professional success. The widespread global interest...
Joydeep Ganguly, who is currently Senior Vice President, Corporate Operations, at Gilead Sciences, Inc., places a premium on working for a mission-oriented company. “If you choose the right company with the right ethos and a deep, tangible commitment to the patients, the mission is not just rhetoric,” he said. “You see it in the way they do things. If you work for a company that makes that...
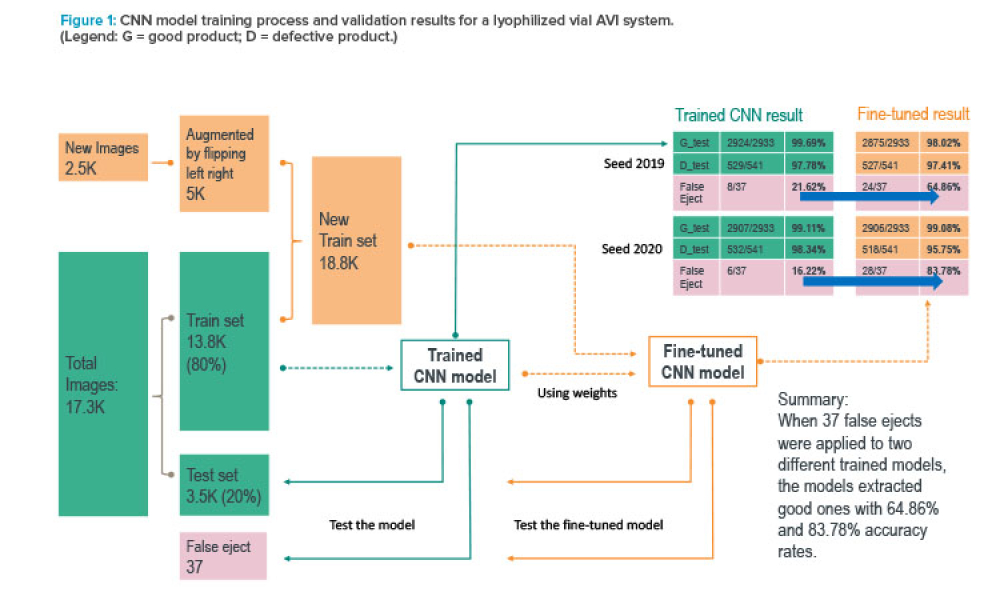
Pharmaceutical companies rely on automated vision inspection (AVI) systems to help ensure product safety. Although these systems overcome challenges associated with manual inspection, they can be hindered by limitations in their programming—if the system is programmed to consider every variation in inspection conditions, it is likely to falsely identify defects in safe products. This article...
Oligonucleotides are a relatively new class of drugs, composed of natural and synthetic nucleotides, which primarily include small interfering RNA (siRNA), micro RNA (miRNA), and antisense oligonucleotide (ASO). These molecules achieve therapeutic effects through RNA interference, degradation, or splice-modulating pathways.
ISPE is celebrating its 40th anniversary this year! Here are some of the memorable events from the last 40 years.
As Pharma 4.0™ increasingly becomes reality, our validation practices must change. We can no longer apply 20th-century thinking to 21st-century technology and resources. Validation must adapt to industry shifts from iterative to...
In many critical ways, the design of facilities for multiple cell therapy processes is unlike the design of conventional pharmaceutical facilities. This article surveys several of the key issues to consider when designing facilities capable of manufacturing multiple cell therapies, including regulatory definitions, product life cycles, processing systems, relevant cell therapy technologies and...
The current regulatory framework in the pharmaceutical industry places pressure on marketing authorization holders (MAHs) to demonstrate quality oversight, and a systematic risk management process is a prerequisite for avoiding compliance and productivity pitfalls. This article focuses on options to improve day to- day operations and to ease decision-making by integrating operational risk...
Designing new facilities for cell and gene therapy manufacturing is a challenging task given the many uncertainties in this industry sector, including varying potential demand for any given new therapy, evolving platforms and technology, questions about equipment reliability, learning curves for analysts and operators, possible sourcing issues, and variable lead times for key raw materials....
Existing risk-based approaches to computerized system compliance and validation as outlined in GAMP® 5
The ISPE France Affiliate is fortunate in many ways. The pharmaceutical industry in France is world class, employing close to 100,000 people and generating €55.9 billion in annual revenue.
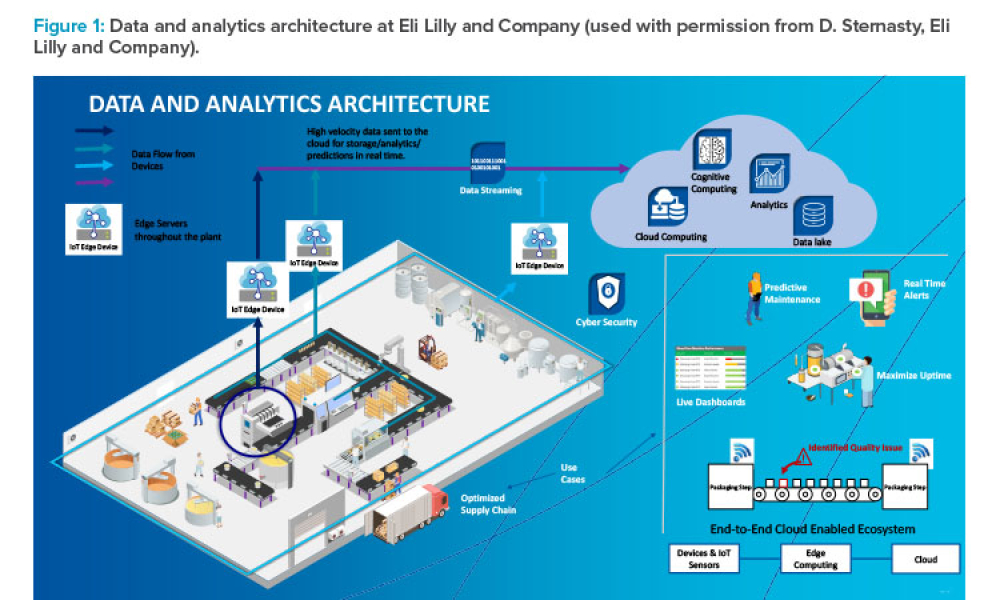
In the pharmaceutical industry, digitalization involves developing and implementing digital technologies at all levels of pharmaceutical operations. The aim is to transform the industry by capturing, analyzing, and using vast amounts of data collected from a wide range of sources to support research and development (R&D), clinical development, drug manufacturing, supply chain management,...
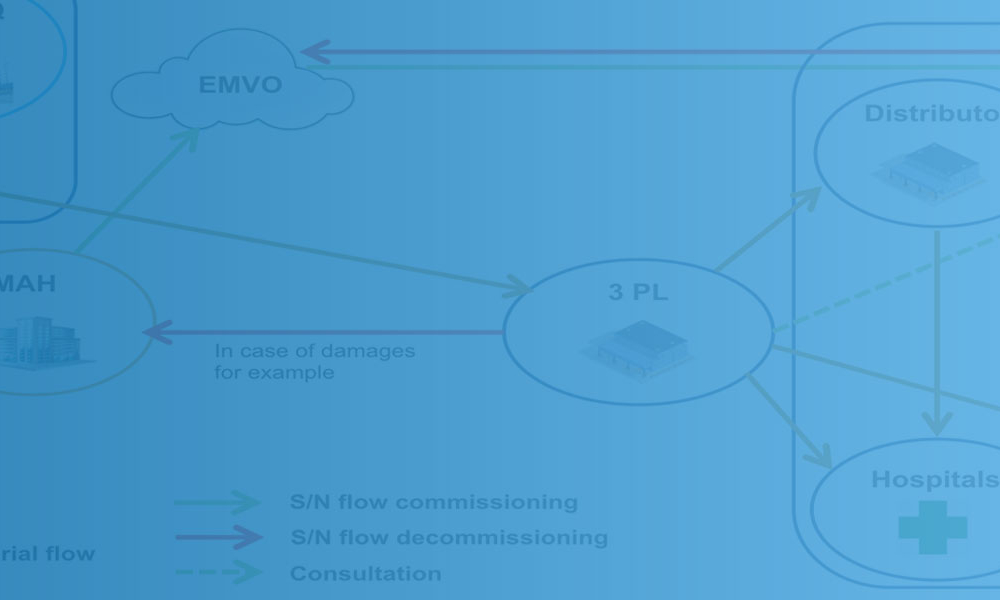
Recent projects on serialization and track and trace help illustrate the concepts of vertical and horizontal integration. With vertical integration, the unique product identification information (serial number, lot, etc.) used by sensors and printers on the packaging lines is made accessible to the supply chain and regulatory hubs throughout the entire technology stack. With horizontal...
The 2020 ISPE Aseptic Conference was capped off on Tuesday, 3 March, by the Interactive Regulatory Panel session, which has been a popular feature of the conference during its 29 years. The session featured a panel...
For over two decades, the ISPE Barrier Isolator Survey has gathered meaningful data on the applications of barrier technology and been a resource for the fill-finish pharmaceutical industry community. This article provides context for the latest survey, the first in several years, and presents its key results, which were first shared at the
Pam Cheng is Executive Vice President, Global Operations & Information Technology, at AstraZeneca, a United Kingdom–headquartered pharmaceutical company with more than 60,000 employees. In this role, she combines her expertise as an engineer with business savvy and seeks opportunities to lead her company and her industry forward in innovative ways.
As the old saying goes, “Time is money.” In today’s industrialized world, this adage is profoundly true. Manufacturers can no longer afford to overlook operational excellence. A new production philosophy called “Lean manufacturing” has been developed to save as much time as possible during manufacturing processes. In some industries, such as the automotive sector, Lean has almost been...
At the 2019 ISPE Global Pharmaceutical Regulatory Summit, regulators updated attendees on approaches to industry innovations and the ongoing work on harmonization and reliance around the...
Virtually every ISPE member has at least one story to tell about how health authority inspections or the review and approval of regulatory applications have affected their efforts to supply critically needed medications to patients globally. Although these stories may emphasize the considerable challenges that ISPE members face, they also frequently identify great opportunities for innovation...
Annex 2 is the Good Manufacturing Practices (GMP) document by the Pharmaceutical Inspection Co-operation Scheme (PIC/S) addressing manufacture of biological medicinal substances and products for human use. This article shares information about Annex 2 and ISPE’s submitted comments to the draft revision of Annex 2 that was released for public consultation in September 2019.
On 20 November 2019, the ICH Assembly endorsed the Q12 guideline, “Technical and Regulatory Considerations for Pharmaceutical Product Lifecycle Management,” at its biannual meeting in Singapore. This transformational guideline has a wide scope of applicability across pharmaceutical drug substances and products (both chemical and biological), drug-device combination products that meet the...
This article introduces the concept of robotic process automation (RPA) and discusses how the technology may be used within a GAMP® framework to support both non-GxP and GxP processes.
In this issue of Pharmaceutical Engineering®, we address an array of sustainability topics. This article surveys topics that will likely have a significant global impact on the way we conduct our business over the coming decade. We trace some history of sustainability in the life sciences industry and identify future issues of concern, including a number of areas...
The article appraises the real-world experiences of two pharmaceutical companies approaching the rollout of energy- and water-reduction programs to selected facilities around the world. It is the result of more than two years of collaboration between the company corporate teams, individual site teams, and an external specialist consultant.
Sustainability is a key principle for pharmaceutical companies in 2020. However, translating corporate goals into meaningful improvements can be a challenge, particularly when competing factors such as complex technical requirements or ambitious project schedules are involved.
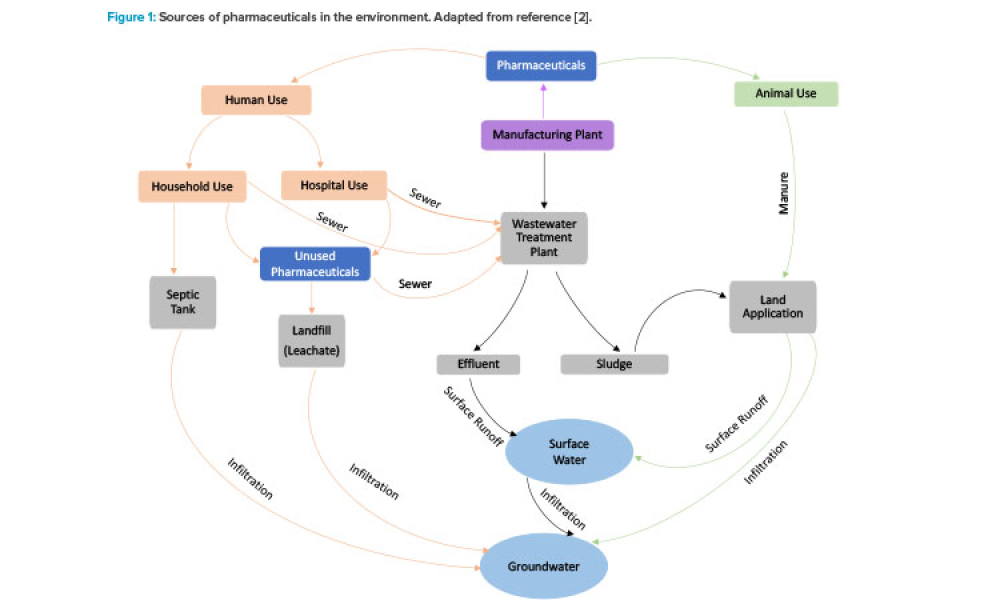
Medical treatments and pharmaceuticals are indispensable in improving quality of life. In recent years, however, pharmaceutical compounds have become a significant group of environmental pollutants, shown to pose risks to human health and have adverse environmental effects.
With the start of the new year, Pharmaceutical Engineering® is launching a new series of profiles of industry leaders. This ongoing series will look at the lives and careers of individuals who are changing the face of the pharmaceutical industry.
Ranjana B. Pathak, BSc (Hons), MBA, DHA, has spent nearly 40 years in the pharmaceutical industry. Currently the President and Global Head of Quality, Medical Affairs, and Pharmacovigilance at Cipla Ltd. in Mumbai, India, Pathak’s long tenure has afforded her an informed perspective on the past, present, and future of the industry. An active member of the
Every important cause needs its champion. Champions have a vision of how things should be, and a passion to reach their goals. They are committed and determined to achieve positive results, are willing to do the heavy lifting, and will take consistent and massive action until results are achieved.
Since the early 1990s, when the “upstart” biotech industry realized that its future success would be heavily influenced by the ability to manufacture multiple products within the same facility,
Applying emerging technologies can lead to more robust and flexible manufacturing processes that in turn can help the pharmaceutical industry respond to drug shortages, reduce interruptions in production and delivery of medicines, ensure consistent clinical performance of products, and achieve other benefits. Although some may believe that regulators are averse to the use of emerging...
As the pharmaceutical industry balances demands for small-batch and blockbuster products and encounters new regulations, there is a need for efficient and safe production technologies that can meet stringent quality and safety requirements for the aseptic filling of drugs. Looking forward, manufacturers should anticipate future format, packaging, and filling needs, and seek technologies with...
To meet the EU serialization deadline on 9 February 2019, pharmaceutical companies and their contractors have had to reorganize their manufacturing lines and logistics to ensure compliance with the EU’s Falsified Medicines Directive (FMD) of 2011 and the EU Commission Delegated Regulation 2016/161 of 2016. Worldwide, other anticounterfeiting regulations are already in place or coming soon in...
Great strides are being made in bioprinting, and the end result could revolutionize pharmaceutical development and testing.
Great strides are being made in bioprinting, and the end result could revolutionize pharmaceutical development and testing.