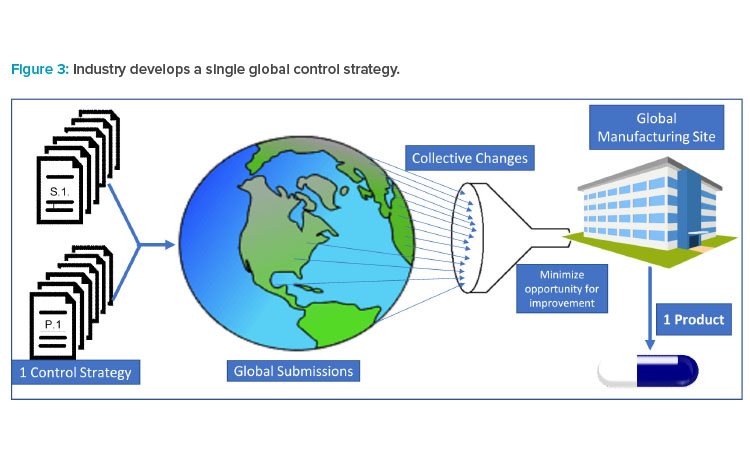
This quarterly newsletter is dedicated to news about ISPE’s regulatory and quality activities. ISPE is committed to fostering communications and interactions to advance common interests among the pharmaceutical industry and regulatory agencies.
The Regulatory Digest Newsletter
ISPE Regulatory Digest 2025

ISPE Regulatory Digest Q4 2025
- The Future of Pharmaceutical Regulation and AI
- Navigating the Challenges of Transportable/Point of Care Manufacturing
- Innovation and Regulatory Harmonization in Latin America: Report on an ISPE Workshop
- ISPE Members Join National Dialogue on Drug Supply Chain Security
- ISPE Regulatory Volunteer Spotlight: PQLI Working Groups Named ISPE 2025 Committee of the Year

ISPE Regulatory Digest Q3 2025
- Navigating through ATMP Guidance and Regulations
- Regulators Discuss Embracing Digital Transformation and Technology in an Evolving Landscape
- Navigating Global Oversight: A Report from the 2025 ISPE China Conference
- The Power of a United Voice: How ISPE Members Can Help Shape Regulatory Guidance

ISPE Regulatory Digest Q2 2025
- EMA on Support for Innovation in Pharmaceutical Manufacturing
- PIC/S on Quality, Capability, Harmonization and Collaboration
- Navigating Innovation: The United Kingdom’s (UK) Evolving Regulatory Landscape
- ISPE Members Continue Training for Health Authorities on ICH Q12 Implementation
- A Strategic Approach to Regulatory Surveillance and Advocacy

ISPE Regulatory Digest Q1 2025
- Regulator Expectations for Data Management in Sterile Manufacturing
- Approaches to Lifecycle Management and Regulatory Readiness in Advanced Therapies
- Elevating Pharmaceutical Quality: FDA’s Office of Quality Surveillance and ISPE’s APQ™ Program
- FDA on Pharmaceutical Compounding
Previous Regulatory Digest Newsletters
.jpg)
ISPE Regulatory Digest Q4 2024
- FDA on Regulatory Reliance and Agility
- EMA on the Future of Innovation and Regulation
- ISPE Global Regulatory Town Hall: The Future is Now
- Global Adoption Status and Implementation of ICH Guidelines Q12, Q2(R2), and Q14
- Roger Nosal awarded ISPE Distinguished Achievement Award
.jpg)
ISPE Regulatory Digest Q3 2024
- FDA on AI in Pharmaceutical Manufacturing
- A Holistic Approach to Supply Resiliency
- ISPE Regulatory Perspectives in Pharmaceutical Engineering® Magazine
- Quality Management Maturity Industry Study

ISPE Regulatory Digest Q2 2024
- ISPE Issues Report on Barriers to Innovation
- International Regulatory Dialog at 2024 ISPE Europe Annual Conference
- Annex 1 Implementation a key topic of 2024 ISPE Aseptic Conference
- Regulatory Concerns in Asia-Pacific

ISPE Regulatory Digest Q1 2024
- Quality Beyond CGMP: FDA Initiatives
- Advancing Digitalization in Manufacturing: EU Initiatives
- Regulators Discuss AI and its Effects on Regulatory Issues
- Regulatory Challenges and Reform in Asia-Pacific
ISPE Regulatory Digest Q4 2023
- ISPE President/CEO fireside chat with FDA Commissioner
- Health Canada on Advanced Manufacturing and Other Trends
- Review and inspection practices spotlighted at Global Regulatory Town Hall
- ISPE’s regulatory and quality initiatives deliver conference sessions, articles, and training
ISPE Regulatory Digest Q3 2023
- EMA Executive Director on the Value of Adaptation, Trust, and Collaboration
- FDA Office of Quality Surveillance on Quality Management Maturity
- EMA on International Cooperation, Harmonization, and Reliance in GMP
- Industry-regulatory discussion panels
ISPE Regulatory Digest Q2 2023
- Barriers to Innovation: Your Input Requested
- ISPE Releases Drug Shortages Prevention Model
- Report on ISPE Work to Support EU HERA
- ISPE Comments on Draft Guidelines
Regulatory Digest Q1 2023
- ISPE Launches Initiative on Enabling Global Pharma Innovation
- Annex 1 Implementation from the Regulators’ Point of View
- Bob Tribe, ISPE Asia-Pacific Regulatory Advisor, Retires
- Your Expertise Can Shape Regulation and Guidance
Regulatory Digest Q4 2022
- ISPE’s Advancing Pharmaceutical Quality (APQ) Program Fully Launched
- ISPE’s APQ Program presented at FDA Advisory Council Meeting on Quality Management Maturity
- ISPE Highlights Pharma 4.0 at PIC/S 50th Anniversary Event
- ISPE Holds Regulatory Summit on Modernizing Inspections
- ISPE Singapore Affiliate Regulatory Panels
- Regulatory Insights from 2022 ISPE Annual Meeting
Regulatory Digest Q3 2022
- Are you ready to implement ICH Q9(R1)?
- ATMP Regulations: Comparison of EudraLex Part IV with PIC/S Annex 2A
- FDA’s Approach to Advancing Quality Surveillance
- EMA on Facilitating Innovative Manufacturing
- Continuous Manufacturing of Drug Substance vs Drug Product
- ISPE’s Asia-Pacific Regulatory Group Re-established
- Quality and Regulatory Challenges, Learnings, and Future Opportunities: ISPE 2022 Annual Meeting
Regulatory Digest Q2 2022
- Regulator/Industry Panels at 2022 ISPE European Annual Conference
- ICH Q2(R2) and Q14 aim to promote more robust analytical processes, greater ease of switching analytical methods post approval
- ISPE Helping Industry Prepare to Implement ICH Q9 Revision
- ISPE Assists Health Canada with ICH Q12 Training
- ISPE’s Advancing Pharmaceutical Quality (APQ) Program Third Guide Released
Regulatory Digest Q1 2022
- Industry Study Finds Divergence in Regulators’ Interpretation of ICH Guidance Related to Control Strategies
- Senior US Pandemic Advisor on Messaging and the Public’s Faith in Pharmaceutical Products
- Path to a Global Dossier: a glimpse of the future?
- Global Regulators Discuss Data Integrity and Real-World Evidence
- ISPE’s Biotechnology Conference and Workshop Featured Several Panel Discussions with Global Regulators
Regulatory Digest Q4 2021
- US FDA and WHO Highlight Agility and Collaboration at 2021 ISPE Annual Meeting & Expo
- Regulators Answer Industry’s Top Questions at 2021 ISPE Annual Meeting Global Regulatory Town Hall
- Regulators Discuss Contemporary Approaches to Drug Shortage Prevention
Regulatory Digest Q3 2021
- Global Regulators and Industry Discuss Shared Responsibility for QRM at ISPE June Summit
- Expanded Coverage of ISPE’s Summit on Remote/Distance Assessments
- ISPE’s PQLI® Initiative helps advance new Regulatory and Technology Approaches
- ISPE’s APQ Program: Management Responsibilities and Review Guide Released
- ISPE Initiatives Support Supply Chain Resiliency
Regulatory Digest 2021 – Q2
- Global Regulators Discuss Remote/Distant Assessments, Audits, and Guidance at ISPE Summit
- ISPE and an Association Group address Annex 1 Implementation Timelines
- ISPE Commences Study on Business Continuity Planning for Drug Shortage Prevention
- Integration of QRM and KM Provide Opportunity for Effective Risk Management
Regulatory Digest 2021 – Q1
- ICH Q12 Implementation Strategies Discussed During Webinar on Challenges and Success of ICH Q12
- 2020 ISPE Annual Meeting & Expo: How Regulators are Responding to COVID-19’s Impact
- Regulatory Panel Addresses COVID-19 Challenges
- Regulators Discuss Distant Assessments During and After COVID-19
- Singapore Affiliate’s 20th Anniversary Conference
- ISPE Singapore Affiliate Interviews HSA’s Sia Chong Hock
- Remote/Distant Assessments, Audits, and Regulatory Guidance
- FDA Commissioner on Lessons Learned from COVID-19
- ISPE Participated in DAF ACT Initiative to Support Domestic Manufacturing of API
- ISPE’s Advancing Pharmaceutical Quality Program Launched
- Drug Shortages: Looking Forward with Regulator Insight
- Business Continuity Planning to Prevent Drug Shortages
- ANVISA training facilitated by ISPE’s Latin America Regulatory Group
- ISPE’s Regulatory Committees, Working Groups, and Initiatives



.jpg)
.jpg)