Creating Effective Standard Operating Procedures
Personnel management is the most challenging variable in maintaining current Good Manufacturing Practice (cGMP) across the life cycle of drug manufacture, safety, and supply. A standard operating procedure (SOP) outlines agreed-upon instructions for personnel training and instructions for maintaining systems, machines, documents, and records in a qualified state to produce safe products. This article explores the role of standard operating procedures, as well as their structure and components.
The need for succinct, well-written, and focused standard operating procedures is best illustrated by examples. The following are gleaned from our experience, from design to compliance, helping companies respond to adverse regulatory findings by the US FDA, EMA, and others.
In one case, a lengthy and unclear standard operating procedure was ignored and the “best operator” was “training” others in vial capping procedures. The spring pressures applied to dies on a vial capping machine were observed to be variable and the dies mismatched. The operator compensated for this mismatch with trial-and-error adjustments, and the trainees learned unqualified methods.
In cases where such practices survive regulatory inspections, this may enshrine the belief that they are compliant with regulations. However, when inspectors issue their reports, their lists are not comprehensive and may include only the most egregious issues found. Even though the inspectors may not have listed concerns about the vial capping procedures, the procedures were not cGMP compliant and increased patient risk.
A developing trend is for corporations to generate corporate standard operating procedures for use as site standard operating procedures. An often-stated justification for the practice is that it limits the number of standard operating procedures, which is supposed to make the standard operating procedure update process easier. However, the practice may blur the distinction between corporate documents and site-specific standard operating procedures and lead companies to stray from cGMP.
For example, when a company included as many dependent procedures as possible in a standard operating procedure, the result was an unwieldly, inefficient calibration standard operating procedure. The standard operating procedure encompassed multiple analytical and nonanalytical subsystems, and some types of calibration were understood by personnel to be the domain of certain departments, even though this was not stated in the standard operating procedure. Because many departments and systems were included in a single standard operating procedure, those tasked with performing specific activities had the unnecessary responsibility of remembering the standard operating procedure’s nuances and exceptions. Regulators looking at these kinds of standard operating procedures may rightfully question the efficacy of training, especially when the duration of training is too short to plausibly learn the documented procedures.
Standard Operating Procedure Building Blocks
To be most effective, standard operating procedures should be succinct, intuitive, easy to navigate, traceable, and regularly approved. Concise standard operating procedures greatly help users and provide assurance to regulators that procedures are controlled and compliant.
To ensure compliance and traceability to a qualified state are achieved, companies should make approved standard operating procedures traceable and confirm they have an audit trail. Appointing a single individual as owner of approved standard operating procedures further strengthens control over them. When this does not happen, original ap-proved documents may be lost or untraceable.
Revisions should be made only when changes occur to the process or the procedural steps, or when a review is compulsory. Nonprocedural changes—such as inconsequential typographical errors and logo changes—should be noted by the standard operating procedure owner and only added to standard operating procedures during subsequent revisions.
Standard operating procedures should be hard copies or noneditable files that are controlled and archived in a secure location. Although editable files such as Microsoft Word documents may be used and circulated prior to approval, they are not suitable media for approved documents.
To generate a standard operating procedure or revise a legacy standard operating procedure to be as effective as possible, the authors of the standard operating procedure should use clear wording, break down content into parent and child documents as needed, use detailed work instructions when necessary, include engineering references and images for clarity, and follow a defined, easy-to-use structure.
Clear Wording
The apparent simplicity of high-quality standard operating procedures belies the effort and cost of producing and editing them. When companies spend insufficient time editing and producing standard operating procedures, wordy and confusing documents are a likely result. For instance, standard operating procedures may include awkward, repetitive text because they were hastily completed in an effort to close corrective and preventive actions (CAPAs) and authors inserted partial transcription related to regulatory (FDA, EMA, etc.) observations. During follow-up visits, inspectors may be impressed by seeing the exact CAPA wording in the standard operating procedure, but the insertions can be counterintuitive or ineffective for those who are expected to adhere to the procedures. Staff training can suffer as a result, leaving personnel dependent on heuristic learning from the “best operator.” Consequently, operations can resemble trade practice instead of qualified procedural methods.
Parent and Child Standard Operating Procedures
To prevent standard operating procedures from becoming bloated, one solution is to adopt independent parent standard operating procedures, child standard operating procedures, and annexures. For instance, a preventive maintenance standard operating procedure can be broken down into child standard operating procedures for consumables, equipment, buildings, and so on, or a calibration standard operating procedure can have child standard operating procedures or other types of attachments for calibration masters, analytical subsystems, nonanalytical subsystems, etc.
The advantage of using a parent document and child documents is that when subcategories change or need to be revised, the focus is limited to discrete standard operating procedures or attachments. Consequently, retraining cost is lower because it is specific to the subcategory rather than the standard operating procedure in general. As standard operating procedures become more succinct, they become easier for staff, auditors, and regulatory inspectors to understand and explain.
Work Instructions
Using work instructions to provide detailed step-by-step instructions to operators on a separate document, instead of in the standard operating procedure proper, can be effective. The standard operating procedure can provide general information, and the respective work instructions can address the details.
This approach is especially useful when the language of the standard operating procedure and its associated documents is not in the native language of operators. Although only one version of the work instructions can be regarded as the master file, multiple language translations can be of great benefit. To prevent confusion caused by mistranslation of the master, a note in the translated document should state it is a translation of the official work instructions, and the original document should hold precedence. Revision numbers of the official work instructions and their translated versions must remain the same. Unfortunately, we have seen cases of multiple versions of documents in more than one language, with different instructions.
Engineering References
Another cause of vague standard operating procedures may be the lack of master drawings, such as process and instrumentation drawings and process flow diagrams. In our work, we have found multiple drawings for the same process, computer-aided design files presented as “master drawings,” and different drawings from different departments.
Without a reliable engineering reference, companies may have multiple unrelated drawing revisions indicating different configurations and instrument identifiers, standard operating procedures can become vague, and traceability suffers. To prevent these problems, master drawings should be the responsibility of a designated owner within a department, not, as has been observed, an entire department.
Images
When judiciously used in standard operating procedures, flowcharts, photographs, and diagrams can help personnel understand a process, especially when the standard operating procedure user’s first language is not the same as that of the standard operating procedure. However, overuse and haphazard insertion can lead to fragmentation of text. Images should be annotated to prevent ambiguity.
When judiciously used in standard operating procedures, images can help personnel understand a process.
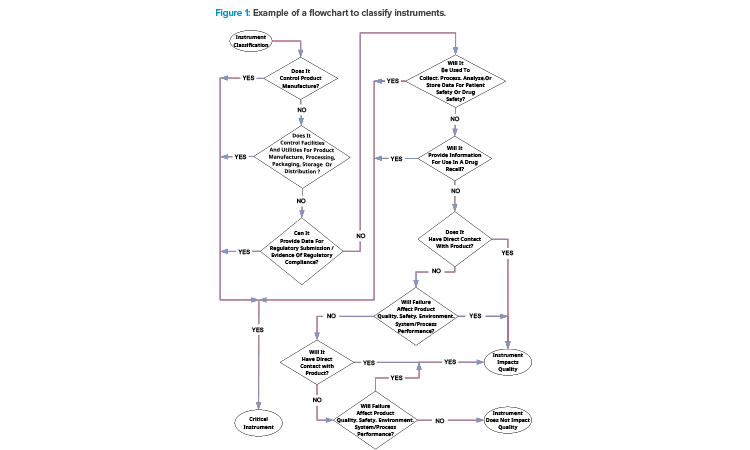
Flowcharts can provide useful overviews of processes. They aid training, and staff can use them as quick reference guides. For instance, a flowchart such as Figure 1 can illustrate the classification of instruments, which has often been a source of confusion in many plants.
Standard operating procedure flowcharts must be carefully edited and reviewed. They can be deceptively difficult to align with the written procedure. It is essential that approved standard operating procedures do not include flowcharts that introduce ambiguity (i.e., the text describes one course of action, but the flowchart indicates another).
| Site details | Document description | SOP no. | Rev. | |
| Title: | ||||
| Supersedes SOP no.: | Issue date: | Review date: | Effective date: | Page X of Y |
| Form number–revision | Page X of Y |
Standard Operating Procedure Contents and Structure
A standard operating procedure generally includes an introduction, background, ownership, instructions, and traceability, all outlined in an agreed-upon format and complete with references, appendixes, and annexures. Although the presentation may differ from the order described here, it must comply with Good Document Practice (GDP). The following standard operating procedure sections are included for completeness; their inclusion in a specific standard operating procedure is a decision for site management.
Provenance
A standard operating procedure’s provenance (its history of ownership and origin) should be included in the header—this will assure users of its validity. At a minimum, the header should include the site details, title, document number, version number, and revision date (see Table 1).
- Site details may include the site or corporation’s logo or printed details.
- Document description can be “Standard Operating Procedure” or “Standard Operating Procedure Appendix.”
- The standard operating procedure number must be unique and comply with a documented numbering system.
- Title is the subject of the procedure. (Note: there is no value in restating the description.)
- Superseded standard operating procedure number is included for traceability. This information is especially helpful when a numbering system changes or the contents of a standard operating procedure change radically.
- Issue date is recorded because standard operating procedures may be issued in advance of the effective date, which is preferable because it allows for an orderly transition and time for training.
- Review date is noted to ensure that a review takes place before a standard operating procedure is no longer valid.
- Effective date is the first day on which a standard operating procedure is valid.
- Every page should show the unique page number and the total number of pages contained in the standard operating procedure (i.e., Page X of Y). This might be included the header, the footer, or both places.
In addition to the page number, the footer may contain the form number (see Table 2). Form number–revision refers to the form identifier used for the SOP, usually with the revision identifier as a suffix. The footer may also contain provision for signatures of approvers if required (not shown in Table 2).
Purpose, Objective, and Scope
The standard operating procedure’s purpose, objective, and scope of systems, equipment, facilities, and/or process are best described in the introductory sections of the document.
- Purpose outlines the qualified processes, equipment, or systems activity used in maintaining cGMP for which the SOP was developed. It should indicate the user and any customer requirements, and identify the site owner.
- Scope identifies the target department (or departments) of the standard operating procedure, their locations, and the personnel to whom the standard operating procedure applies. For clarity and to exclude ambiguity, a description of items not within the scope should be included.
- Objective describes the tasks required for each goal of the standard operating procedure and specifies the target process, equipment, utility, or facility. This section should also support the company’s mission statement (and is sometimes called “mission statement”) with respect to the activity for which the standard operating procedure was developed.
Some sites combine the purpose or scope with the objective. However, this format is only recommended when the combined section improves clarity and conforms to an agreed-upon layout.
Approval Signatures
Standard operating procedures must include an approvals section or page where owners can append their signatures and the date of their signing. Some companies require the author, reviewers, and approvers to sign every page, even when the standard operating procedures are lengthy. As a result, some standard operating procedures that we reviewed had more than 200 signature/date insertions. Signing every page is generally unnecessary. Instead, consider using only initials on individual pages, or provide signature sections in the front and back sections of standard operating procedures to bracket their contents.
Table of Contents
A table of contents helps users locate relevant sections, which is particularly useful during an inspection or audit. Most writing software can automatically generate the table of contents.
Definitions and Abbreviations
A definitions section clarifies any unfamiliar terms or jargon for the reader. It is especially useful when auditors and regulatory inspectors review procedures.
All acronyms or abbreviations should be defined. This may be done in a list or by enclosing the acronym or abbreviation in brackets and displaying it immediately after the spelled-out term is presented in the text.
Roles and Responsibilities
A roles and responsibilities section identifies the duties of the author, owner, reviewer, technicians, operators, management staff, and quality assurance. For situations in which technicians or operators are not staff, reference to the relevant standard operating procedure for contractors should be given.
Procedure
The procedure section should outline the process and enumerate the steps necessary to accomplish tasks. As noted previously, if there are many steps in a procedure, consider including only the main content of the procedure and reserving details and specifics for child standard operating procedures and other addenda.
Revision History
A history of standard operating procedure revisions must be included for traceability. Such a history is easily maintained if the parts of the standard operating procedure (sections, paragraphs, subparagraphs, etc.) are comprehensively enumerated for easy identification. Only the history of the most recent revisions, usually the prior three or four, must be shown, provided all other revisions have been archived and are easily retrievable. The company’s approach to tracking standard operating procedure revisions may be noted in its standard operating procedure for standard operating procedures or in the revision history section itself.
References and Related Documents
Relevant references to other documents should be listed in a separate section, as this reinforces the standard operating procedure’s authority. Unfortunately, some standard operating procedure writers will copy references from other documents without assessing their relevance. Unnecessary references should be avoided.
Appendixes and Attachments
Most standard operating procedures have forms, appendixes, addenda, or annexures containing samples of documents or records to be used when executing procedures. These should be used for illustration purposes only and not copied for use as cGMP documents because control over documents would be negated.
Conclusion
Although generating and maintaining standard operating procedures can seem time-consuming, the best standard operating procedures adapt to contingencies without major modifications. The value of producing standard operating procedures that are clear, concise, and intuitive is usually evident when things go wrong, at which time the cost of any corrective action may be greatly magnified.
To avoid standard operating procedure-related problems, companies should consider instituting a program of standard operating procedure revitalization, especially for legacy standard operating procedures. This activity can be conducted by a dedicated team from within the organization, or it may involve the use of consultants. Team members should be experts in an activity covered in the standard operating procedure who are capable of writing in a clear, concise, and intuitive way. Most important, they should write standard operating procedures with the target audience in mind (not only peers or superiors), and peer reviews should be used for technical content.




