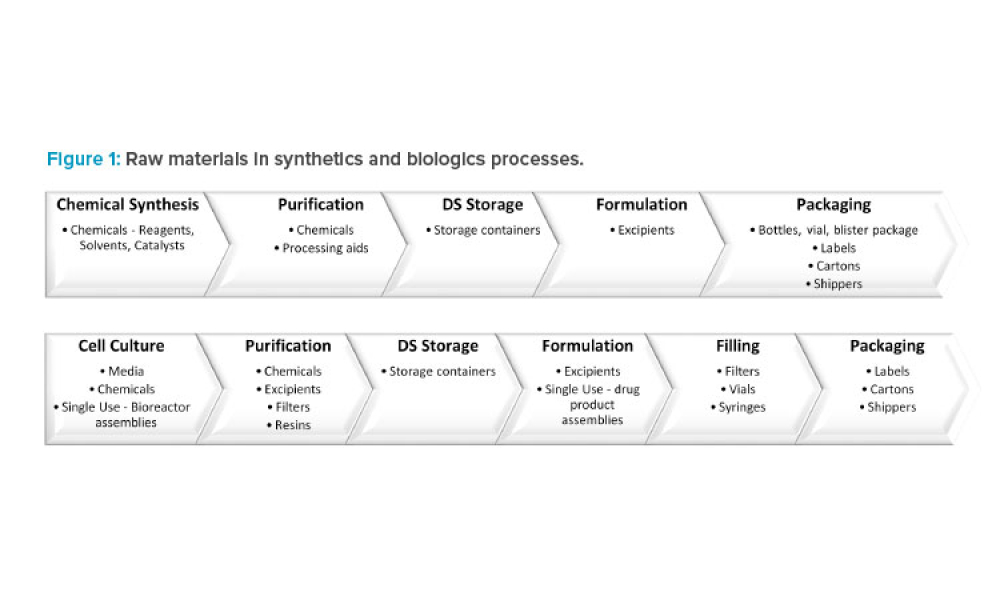
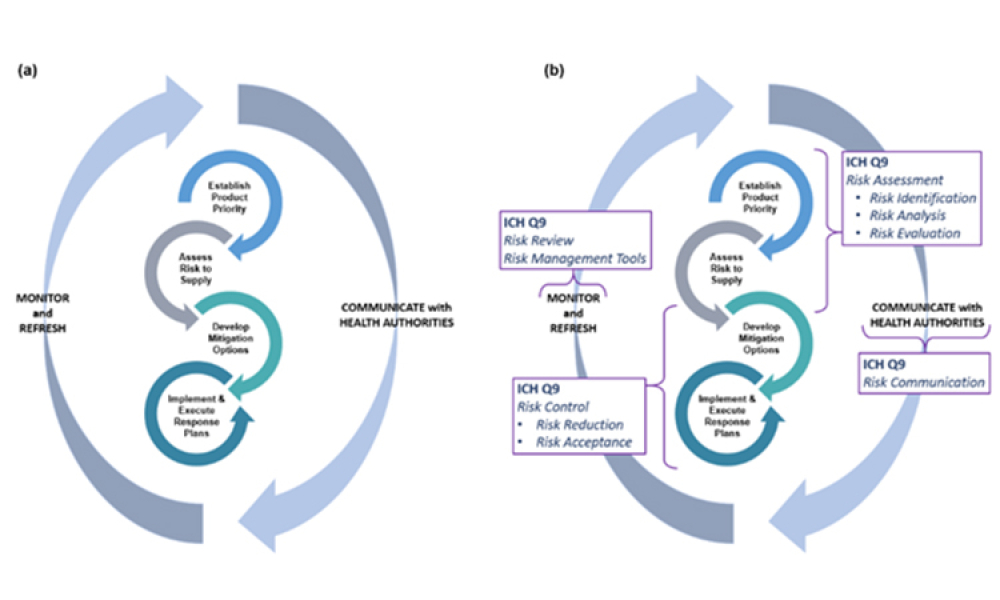
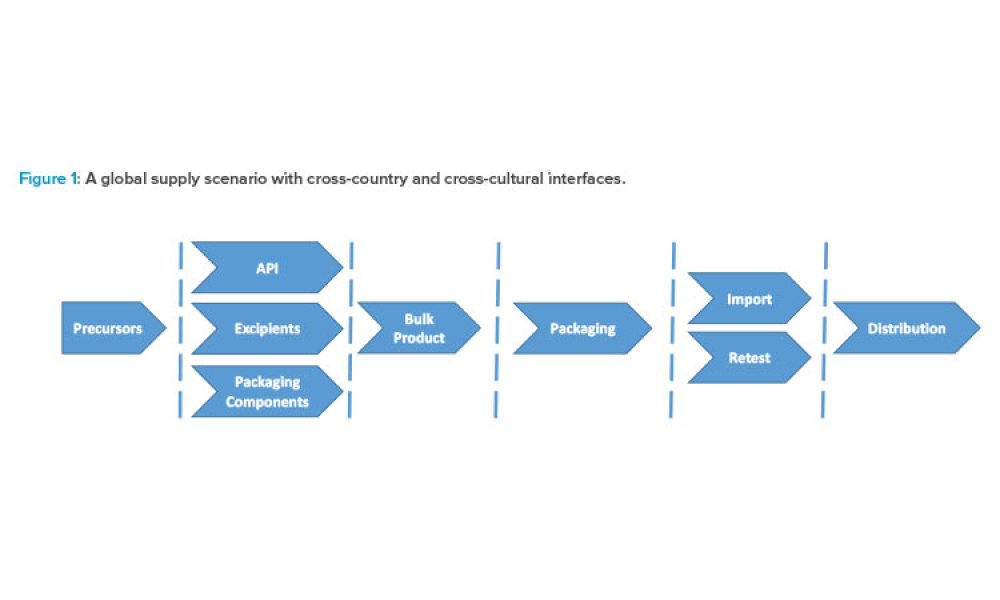
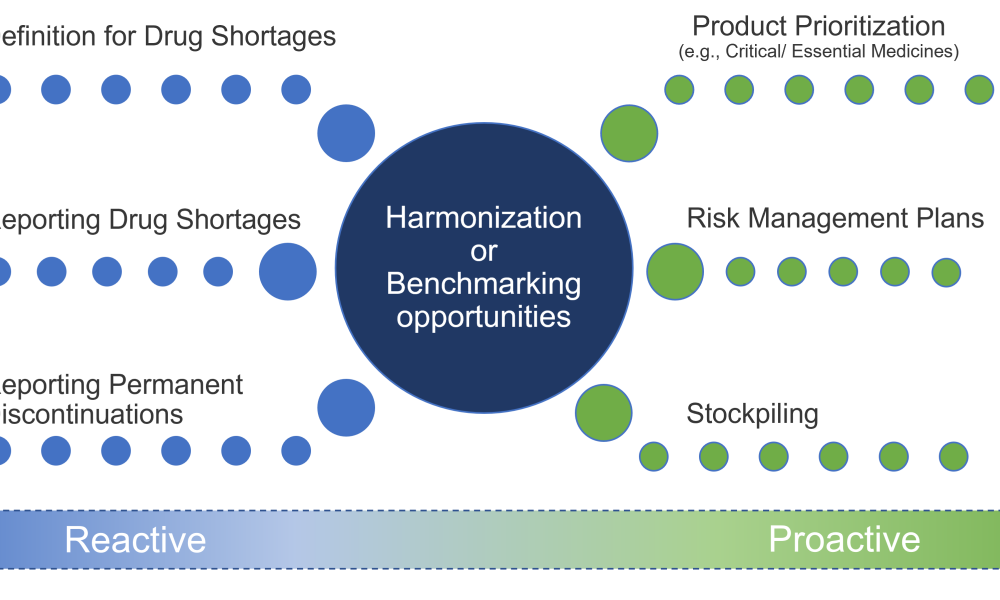
Content focuses on feature key components and the financial impact of supply and distribution chains. Topics focus on the systems required to control and automate the receipt, storage, and dispensation of raw and packaging materials and the storage and distribution of finished products. Other topics could include materials management, operational economics, and warehouse and distribution management.
Join the Conversation
As an ISPE member you can engage with 22 active CoPs, including new communities focused on Artificial Intelligence and Sustainability. Connect with experts and join the conversations: Become an ISPE member
ISPE members: Get more involved by volunteering.
Guidance Documents
Active Pharmaceutical Ingredients (1)
+Drug Shortages (1)
+Investigational Products (1)
+Packaging (1)
+Project Management (1)
+Regulatory (1)
+Single Use Technologies & Disposables (1)
+Supply Chain Management (6)
+Sustainable Facilities, HVAC, & Controlled Environments (1)
+Community Discussions
Community Discussions
Jul 25, 2025
Data Integrity
Jul 24, 2025
Information Systems
Artificial Intelligence
Data Integrity
Jul 17, 2025
Manufacturing Operations
Oral Solid Dosage
Jul 08, 2025
Pharma 4.0™
Jun 20, 2025
Sustainable Facilities, HVAC, & Controlled Environments
Jun 19, 2025
Quality
Lifecycle Management
Validation
iSpeak Blog Posts
Webinars
Upcoming
On-Demand
ISPE in the News
Latest
-
2025 ISPE Annual Meeting & Expo Focuses on Pharma 4.0™, with Takeda's Gunter Baumgartner as Executive Chair and AstraZeneca's Ciby Abraham as Conference Chair
-
ISPE Training: Advancing Pharmaceutical Quality™ (APQ) Quality Management Maturity Training Course, In Person
-
Report: NIH Cuts, US FDA Delays Could Slow Drug Development
-
Thimerosal to be Eliminated From Flu Vaccines in US
-
US FDA's AI Tool may be Prone to Hallucination
-
Indonesian Military Facilities to Mass Produce Medicines
-
Stevanato Invests $218M in Italy, Indiana Facilities
-
Aenova Adds Spray-Drying Equipment at Ireland Facility
-
"Chromatography decisions made at an early phase of drug development can impact everything from raw material cost and facility design to time-to-market and post-approval changes."
- Importance of Advanced Chromatography Grows
Pharmaceutical Engineering Magazine Articles
White Papers
March / April 2023
As the pharmaceutical industry faces ever-changing global challenges and market forces, it must…

Videos
Professional Development Training
GMP Fundamentals: Holding & Distribution
+Featured Conferences