Celebrating 25 Years of GAMP® Americas

This special anniversary article addresses the history and milestones that define the GAMP Community of Practice (CoP). In celebration of the 25th anniversary of the creation of GAMP Americas, we reflect on the vital role GAMP Americas has played in that journey. We commemorate key accomplishments of its members, share recent activities, and look ahead to the future of GAMP Americas.
Origins of GAMP and Its Guides
The GAMP CoP originated in the United Kingdom in 1991. Led by a team of leading innovators from within the pharmaceutical engineering sector, the forum steering group included David Selby, PhD, then with Glaxo International Quality Assurance; Clive Taylor, then of Wellcome plc; Tony Margetts, PhD, then of Zeneca Pharmaceuticals; and, Annis Bratt, then with SmithKline Beecham. The group was formed in response to US Food and Drug Administration (FDA) inspections that increased focus on the management and controls of automated systems. Under the group’s leadership, industry experts worked to create draft guidance to improve the understanding and communication of regulatory expectations for the use of automated systems supporting Good “x” Practice (GxP) activities. The history of GAMP can be traced through some of the guidance documents created and key milestones shown in Figure 1.
The Supplier Guide, or GAMP Version 1.0 as known today, was published in electronic format in March 1995.1 It addressed expectations from the US FDA and the European Commission’s Good Manufacturing Practice recommendations from Annex 11. The second edition of GAMP followed in May 1996 with new content in response to comments from the European Commission and the US FDA.2 In March 1998, GAMP Version 3.03 was published with funding from ISPE. It included user and supplier guides. Its publication was a foretaste of GAMP becoming a technical subcommittee of ISPE in 2000.
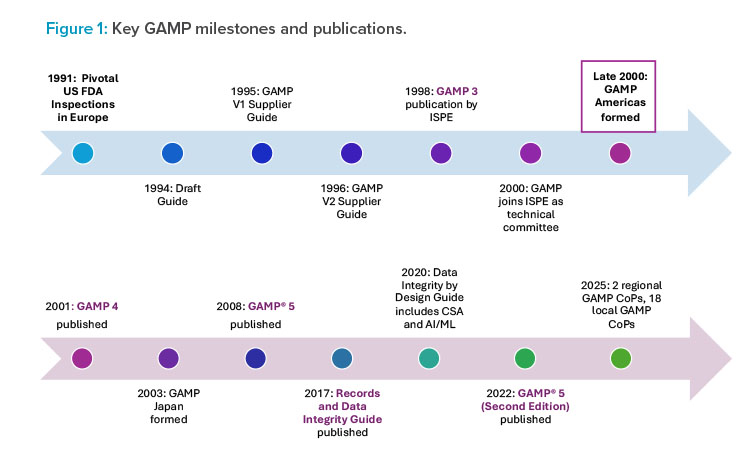
Previously, only pharmaceutical companies were directly involved in writing GAMP guidance. The Supplier Forum was established in 1995 with support from the Medicines and Healthcare Products Regulatory Agency (MHRA). Guy Wingate, PhD (retired, VP & Compliance Officer, GlaxoSmithKline), served as chair of the forum with the goal of providing a community for UK and European vendors, suppliers and consultants, and regulators to discuss the practical application of GAMP guidance. This group was fully incorporated into GAMP in 2000, from which point pharmaceutical companies and vendors would work directly together to write and develop future GAMP guidance.
The ISPE GAMP® 4 Guide for Validation of Automated Systems was released in December 2001 and represented a major revision and new content addressing regulatory and technological developments.4 Key members of the newly formed GAMP Americas—Paul D’Eramo, Arthur (Randy) Perez, PhD, and Rory Budihando-jo—contributed significantly to this updated guide created through a truly international effort, with representatives from sponsor companies, suppliers, and regulators globally. This version of the industry-leading guide broadened the scope to include a wider range of regulated healthcare industries, whereas previous iterations had only focused on systems used for GMP systems. GAMP 4 applied to all regulated systems and included greater focus on user responsibilities and details on the operational phase.
No Longer an Acronym
Prior to the release of GAMP® 4, GAMP had originally been an acronym for Good Automated Manufacturing Practice. With the scope broadened in GAMP® 4 to include much more than just automated manufacturing, GAMP instead became a name, trademarked by ISPE, and the acronym was discontinued. GAMP now represents guidance applicable to computerized systems used in regulated activities covered by Good Laboratory Practice, Good Clinical Practice, Good Distribution Practice, Good Pharmacovigilance Practice, Medical Device Regulations, and GMP. Collectively, these are referred to as GxP.
Taking Technology from Theory to Practice
ISPE GAMP® 5: A Risk-Based Approach to Compliant GxP Computerized Systems was released in 2008, with the GxP in its title reinforcing the broader scope.5 As with all GAMP guidance, this version was created in response to changes in the industry and in regulatory expectations. In particular, the US FDA’s promotion of risk-based approaches and the publication of ICH Guideline Q9 on Quality Risk Management in 2006.6 GAMP® 5 emphasized product and process understanding and offered a practical application of risk-based approaches to computerized systems

Technology and methodology continued to evolve after the publication of GAMP® 5, and GAMP guidance documents have led the way in facilitating industry adoption of innovative solutions and new technologies within the often conservative life sciences industry. For example, GAMP published guidance on using and managing cloud computing and agile methodologies as far back as the 2012 ISPE GAMP® Good Practice Guide: A Risk-Based Approach to Testing GxP Systems (Second Edition).
Computer software assurance (CSA) approaches were written into the 2020 ISPE GAMP® Records and Data Integrity Good Practice Guide: Data Integrity by Design7 which was published two years before the US FDA released it’s draft guidance “Computer Software Assurance for Production and Quality System Software” in 2022, by which time, the full application of computer software assurance (CSA) principles had already been adopted and expanded in the second edition of GAMP® 5. The Guide also includes guidance on blockchain and artificial intelligence/machine learning (AI/ML), providing companies with a framework to leverage these advances into their regulated applications.
The suite of 15 current GAMP® good practice guides has recently been enhanced with an update to the guide on eClinical data. Later this year, ISPE will publish the first-of-its-kind Good Practice Guide on AI/ML technology. It was led and produced by Brandi Stockton, Founder and Managing Partner with the Triality Group, LLC, and Chair of ISPE GAMP Americas CoP Steering Committee.
Recognizing its impact within the life sciences industry, the GAMP Global Steering Committee won ISPE’s Committee of the Year in 2016 and again in 2022 (see Figure 2).
Having originated in Europe, GAMP Americas was established in 2000 as the first GAMP committee outside of Europe, providing a positive step toward global recognition and participation in the GAMP community. The GAMP Americas CoP Steering Committee introduced GAMP principles and methodologies into the US via a forum event, which included keynotes by Selby, Wingate, and D’Eramo.
During the initial GAMP Americas Forum, several special interest groups (SIGs) were formed to begin working on various GAMP good practice guides (e.g., the GAMP Forum Laboratory Systems SIG, and the Global Information Systems SIG). Although these teams started and were led by members of GAMP in the US, and overseen by the GAMP Americas CoP Steering Committee, they were open to and supported by GAMP members throughout the entire international GAMP community.
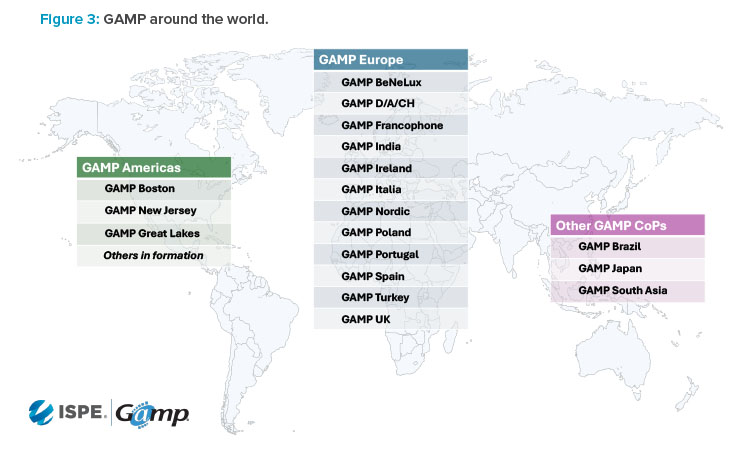
GAMP continued to grow globally through the combined efforts and dedication of GAMP Americas and the original GAMP founders and leaders in Europe, later formalized into GAMP Europe, which today has 12 local CoPs. The 2003 creation of GAMP Japan saw GAMP expand into Asia Pacific for the first time; 20 years later, GAMP South Asia was formed in the region to promote and support GAMP across Australia, Indonesia, Malaysia, New Zealand, Philippines, Singapore, and Thailand. An overview of the GAMP communities around the world is shown in Figure 3.
The GAMP Americas Steering Committee has always maintained a seat for representation from the US FDA. Robert D. Tollefsen, of the US FDA’s Office of Regulatory Affairs (ORA), was an active and valued member of the Americas Steering Committee for many years, providing his insight into discussions and expert review of many GAMP guides. GAMP Americas is now privileged to count Seneca Toms, MS, RAC, of the US FDA’s Center for Drug Evaluation and Research as among its current members.
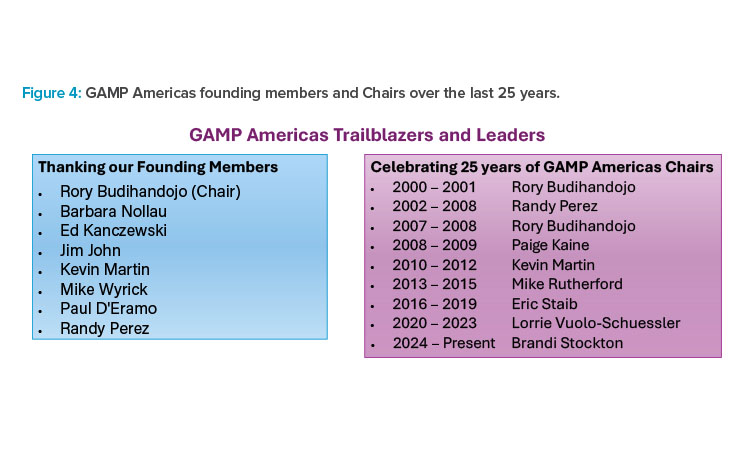
The formation of GAMP Americas and the boundless dedication of its founding and ongoing members have strengthened and expanded the GAMP community through its quarter century of operation, and Figure 4 shows the original committee and the committee chairs through its history.8
GAMP Americas Activities
GAMP Americas has been a very active regional GAMP CoP. Its steering committee has led and/or collaborated with the creation of many GAMP good practice guides. Members of GAMP Americas have also actively participated with other areas of the GAMP community in presentations and workshops during ISPE meetings and conferences.
In their first decades, the GAMP Americas Steering Committee held multiple forums and meetings primarily in the northeast United States. They also held full day fo-rums at ISPE conferences in the United States.
During the last several years, the GAMP Americas Steering Committee has been working to offer more opportunities to GAMP members throughout the Americas by starting local ISPE Chapter GAMP CoPs. These new GAMP CoPs provide local GAMP chapter members with ongoing access to GAMP experts, networking opportunities, and platforms to showcase work and accomplishments, share experience, and cultivate professional growth and visibility within the industry. To date, several new GAMP CoPs have been chartered under GAMP Americas including the ISPE Boston Chapter GAMP CoP, the New Jersey Chapter GAMP CoP, and the Great Lakes Chapter GAMP CoP.
Awards and Achievements
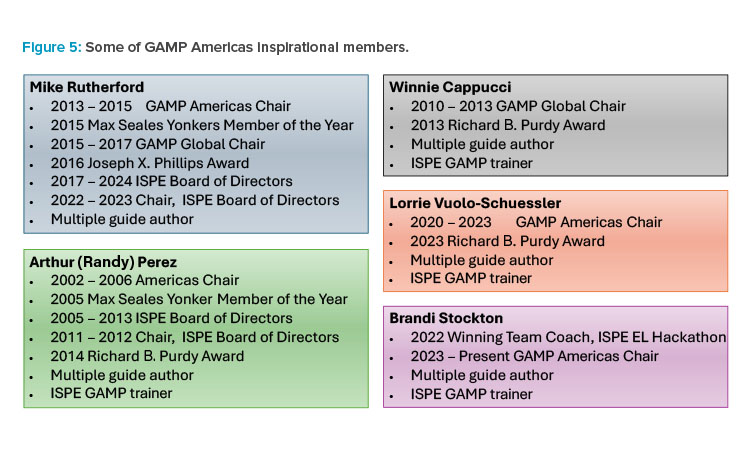
Members of GAMP Americas have also been awarded multiple ISPE international honor awards, including the Max Seales Yonker Award that honors an ISPE member who has made the most significant contribution to the society during the last year, the Richard B. Purdy Distinguished Achievement Award given to an ISPE member who has made significant, long-term contributions to the society, and the rarely given Joseph X. Phillips award that recognizes the extraordinary contributions of its recipients. Among them, GAMP Americas members have been awarded eight ISPE individual honor awards. Figure 5 highlights the achievements of some of the most inspirational members of GAMP Americas.
Cappucci said: “I loved every minute I worked with the GAMPers and still do. What a changing of the guard and a firm foundation for the future.”
Selby reflected recently on GAMP’s continual growth and success from its beginnings in 1991: “It is really impressive to see how the baton has been passed on and enthusiastically taken up by each new generation. Hearty congratulations to all who had the faith to work together in those early days and have continued after me for making GAMP what it is today and so much better than I could ever have imagined.”
Succession and New Members
Membership on a GAMP Steering Committee, Americas or other, has always been based on merit (i.e., demonstrated leadership and/or involvement with GAMP activities through extensive volunteering). Some of our committee members have been investing their own time and expertise into GAMP over decades and their input has been foundational to its success.
New members and new ideas, especially from our industry’s Emerging Leaders, are not only desirable but essential to keep GAMP energized and topical. GAMP Americas has been inspirational in recruiting new steering committee members and engaging with universities, students, and Emerging Leaders, collectively strengthening GAMP’s future.
To encourage succession planning and create opportunities for recruiting new steering committee members, a recent revision to guiding principles applicable to all GAMP CoPs has introduced a limit on the duration of membership on its committees.
Randy Perez and Mike Rutherford: Thank you for your exceptional contribution!
Two of GAMP’s most respected leaders, Randy Perez and Mike Rutherford, are now exiting the Americas Steering Committee under the revised principles. Their volunteer highlights and achievements are vast (see Figure 5) deserving of much more than a few bullet points.
A former senior leader within Novartis and a founding member of GAMP Americas, Perez has continued to devote much of his time to the furtherment of GAMP since his retirement in 2015. During recent years, he has been an active GAMP trainer, acted as an important liaison between GAMP and other ISPE initiatives, and is always willing to share his ideas for GAMP direction and strategy.
Rutherford had an extensive and distinguished career with Eli Lilly and then Syneos Health before retiring in 2023. During his successful executive roles, and continuing after his retirement, he has always made time for GAMP and ISPE. He was instrumental in driving and developing data integrity best practices, many of which are captured in the ISPE GAMP® Records and Data Integrity Guide, which he co-led with Sion Wyn (Conformity Ltd.) and Nigel Price (QCDI Ltd.). No distance was too far, with him sharing his knowledge at ISPE conferences as far as South Korea and Singapore.
Although Rutherford is leaving the steering committee, we know he will continue to be one of GAMP’s strongest supporters. In recognition of the universally high esteem in which he is held within ISPE and GAMP, in September, he was named Interim President and CEO of ISPE and the ISPE Foundation, a post he held until December. Thank you to both for exceptional contributions over the years.
In addition to Perez and Rutherford, there have been many extraordinary GAMP Americas Steering Committee members who have made significant contributions to GAMP Americas throughout the years. Although there are too many to list here, we thank them all for their contributions.
The same succession planning has also happened within the GAMP Global Steering Committee. In addition to Perez and Rutherford, we are also saying goodbye with a heartfelt thanks to Guy Wingate, PhD, (Chair GAMP Council 2000–2010 and ISPE Board of Directors), Chris Reid (Chair GAMP Global Steering Committee 2017–2019 and ISPE Board of Directors), and Chris Clark (Chair GAMP Editorial Review Board 2009–2023). They have all been giants of GAMP and will be missed.
We’ve sadly lost Anthony (Tony) Trill who passed away in August. He was a long-time ISPE member with a decades-long career in the pharmaceutical industry with expertise in Good Manufacturing Practices (GMP) standards. Tony was a major influencer and contributor in the early days of GAMP guidance and deserves much credit for getting European regulators involved with and supporting GAMP.
A Bright Future for GAMP Americas
The knowledge-sharing forums adopted by GAMP Americas and its new local GAMP Community of Practice (CoP) are drawing in attendees both within and outside of current ISPE and GAMP membership. They are attracting new event sponsors and tempting inactive sponsors back after a hiatus of several years. There is new and renewed interest from regulated companies (including supporting chapter/leading committees), increased interest from emerging leaders/students, and increasingly inspiring other ISPE Chapters within the Americas to establish their own local CoPs. The GAMP Boston event achieved a massive level of engagement from younger professionals with approximately 20% of the attendees currently attending school full time.
The GAMP Forums bring much more than just a seminar approach as evidenced by this feedback from one of our members attending our Great Lakes Forum event:
“For me, the ISPE Great Lakes Chapter GAMP Forum in Chicago wasn’t just an educational event, it was a vibrant hub of industry expertise and camaraderie. I met so many fantastic people from the industry, each bringing their unique experiences and insights. This gathering was truly an exceptional experience, blending learning with meaningful connections. The sessions, ranging from critical thinking to the latest in Machine Learning and AI, were not only informative but also inspiring. It’s rare to find an event where you can so deeply immerse yourself in the spirit of innovation and collaborative problem-solving,” said Jahnavi Vellanki, a laboratory systems validation specialist with Labcorp and Great Lakes Forum attendee.
With GAMP events in 2023 in Princeton and Raleigh, and forums in 2024 in Chicago, Indianapolis, and Boston, GAMP’s accessibility throughout the Americas is growing, making it easier than ever to get engaged. There are now links from GAMP Americas to GAMP Brazil, ISPE Mexico, and ISPE Canada Affiliates. The energy in the current GAMP Americas Steering Committee is spreading through its geography.
Any ISPE Affiliate or Chapter in the Americas interested in establishing a GAMP CoP, please contact GAMP Americas leadership.
Getting Involved
GAMP experts around the world are here to help you plan events around digital transformation and to connect you with exactly the right people to help you get involved as a GAMP volunteer.
GAMP topics may overlap with and complement other CoPs. We have experts from our SIGs that can present on many topics with case studies including, but not limited to, software automation and artificial intelligence, blockchain and distributed ledger, data (integrity, quality governance, management), infrastructure, cloud, Agile software development, CSA, and more.
For anyone interested in becoming involved with GAMP:
- Ensure you have selected your affiliate or chapter in your ISPE profile
- Contact your local affiliate or chapter (details can be found on the ISPE affiliates and chapters page)
- Select GAMP CoP in your ISPE profile
- Post into the GAMP community in ISPE Engage—we will respond
- Volunteer for a GAMP SIG or other specific activities: Reach out to any GAMP leader in your region through either the ISPE membership directory or LinkedIn messaging. We are here to help and glad to connect to new enthusiasts
Any ISPE Affiliates or Chapters interested in including GAMP topics at a meeting, please contact GAMP Global or GAMP Americas Leadership (depending on your location). It is vital that all ISPE activities have consistent, aligned messaging around GAMP topics. We are happy to help you do this by recommending speakers, reviewing materials, and promoting your event through our networks, committees, and communities.
- GAMP Americas chair: Brandi Stockton
- GAMP Americas past chair: Lorrie Vuolo-Schuessler
- GAMP Global chair: Charlie Wakeham
More on GAMP
A more detailed discussion of GAMP’s beginnings and the community’s current objectives was published in “Happy 30th Anniversary to the GAMP Community of Practice!” in the May/June 2021 issue of Pharmaceutical Engineering9 written by Siôn Wyn, who has provided exceptional expertise and dedication to the GAMP community for well over 20 years and continues to do so, bringing significant benefit to our industry.
EDITOR’S NOTE: In more recent years, David Selby, worked for Glaxo Wellcome Operations, Barnard Castle, and later Voss, until retiring in 2017. Tony Margetts currently serves as the principal consultant for Factorytalk in Bangkok.






