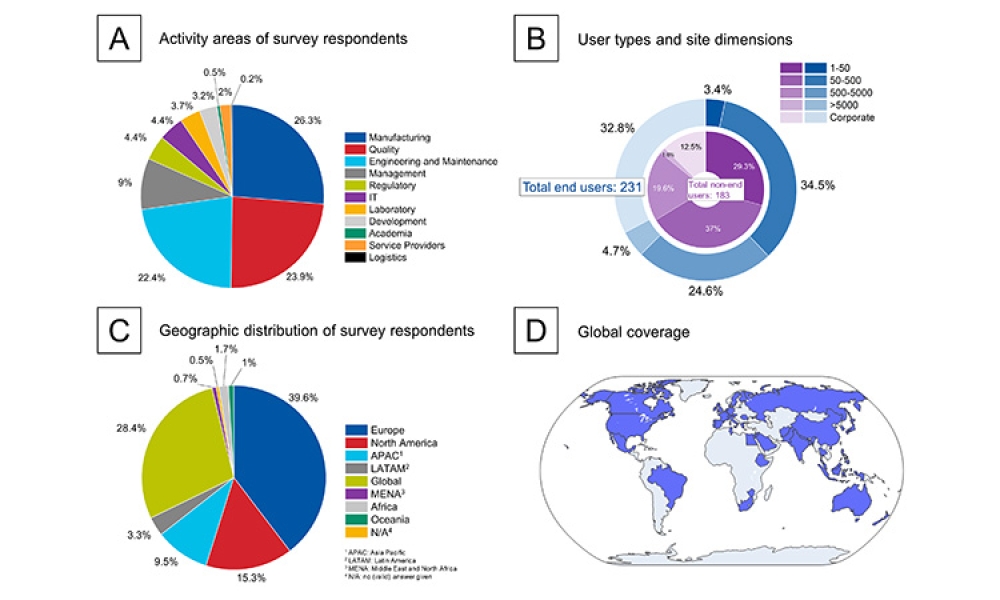
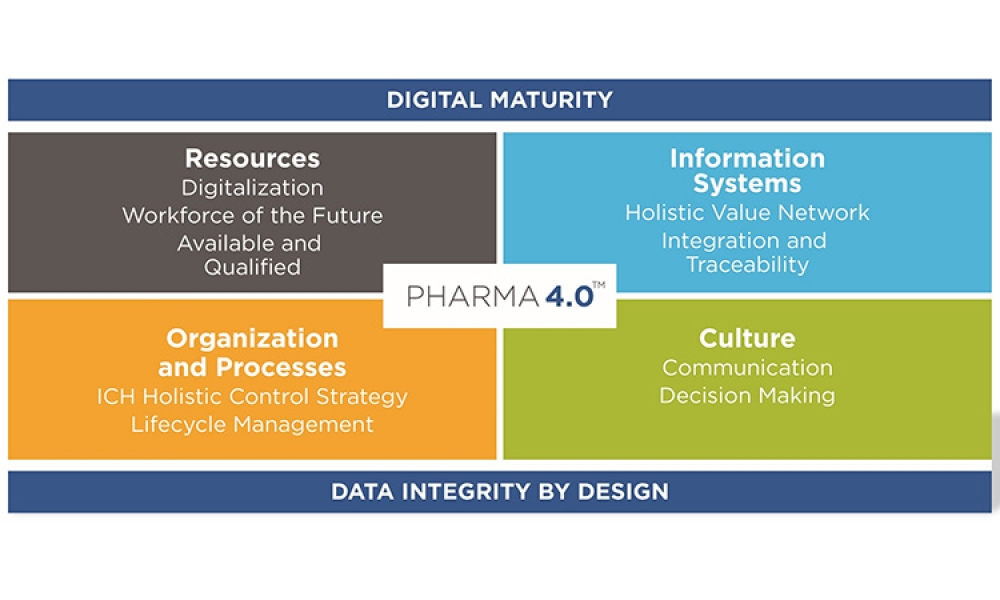
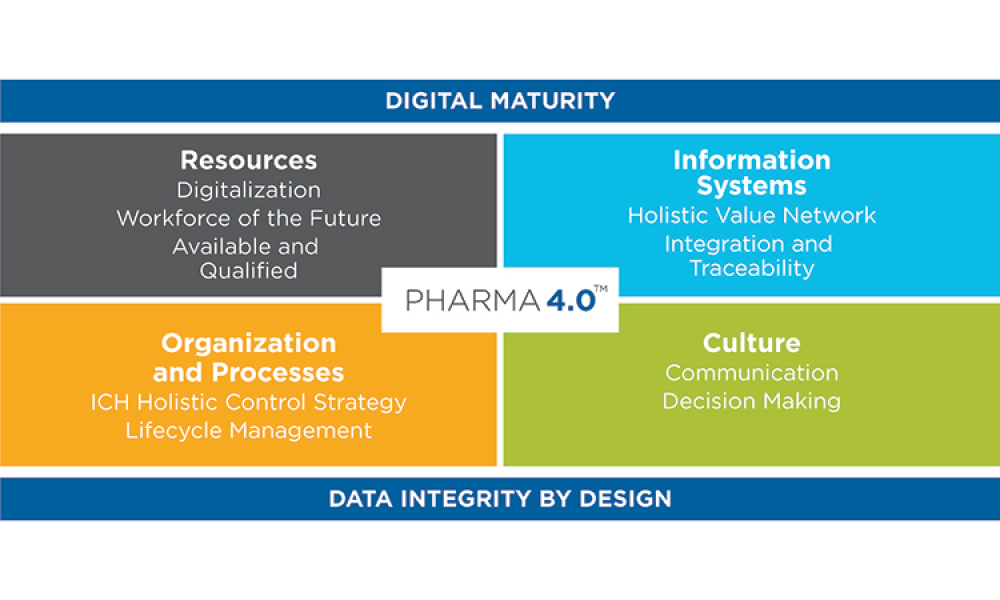
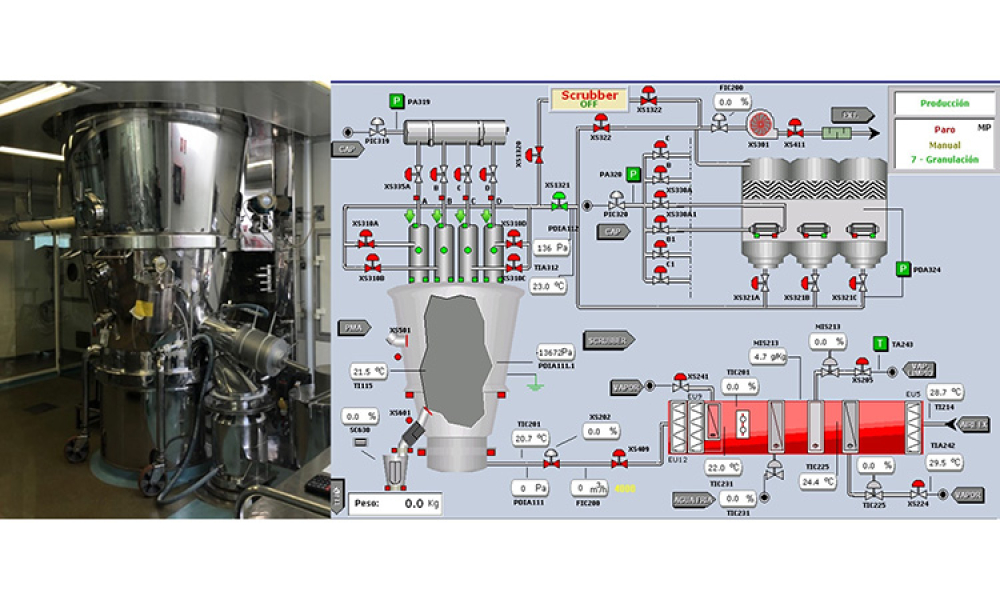
This article summarizes the key findings from the 7th Pharma 4.0™ Survey, conducted in 2023. It explores the demographics of the survey respondents, maturity levels of Pharma 4.0™ adoption, enabling technologies being leveraged, anticipated benefits, and challenges encountered.
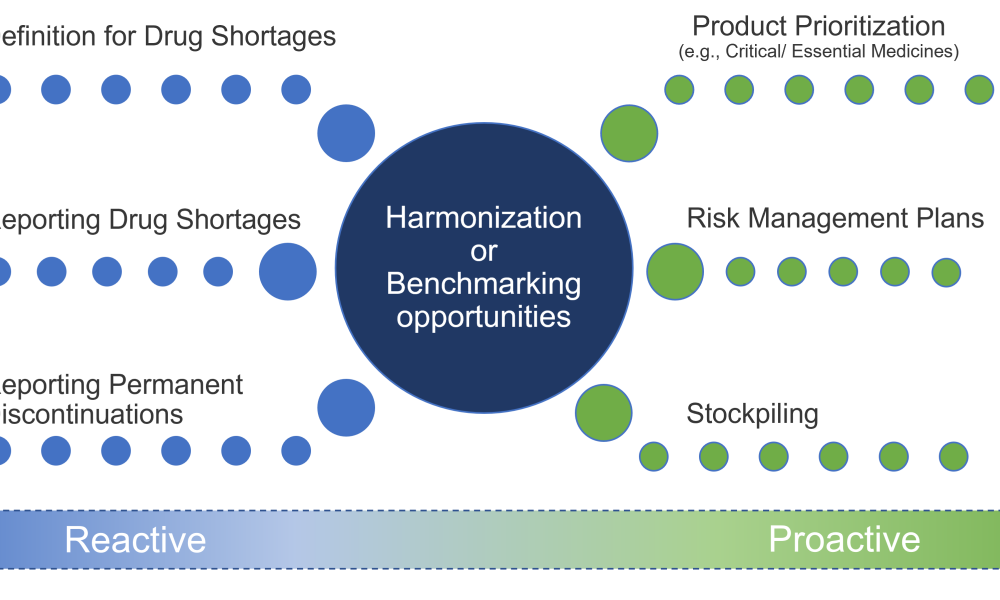
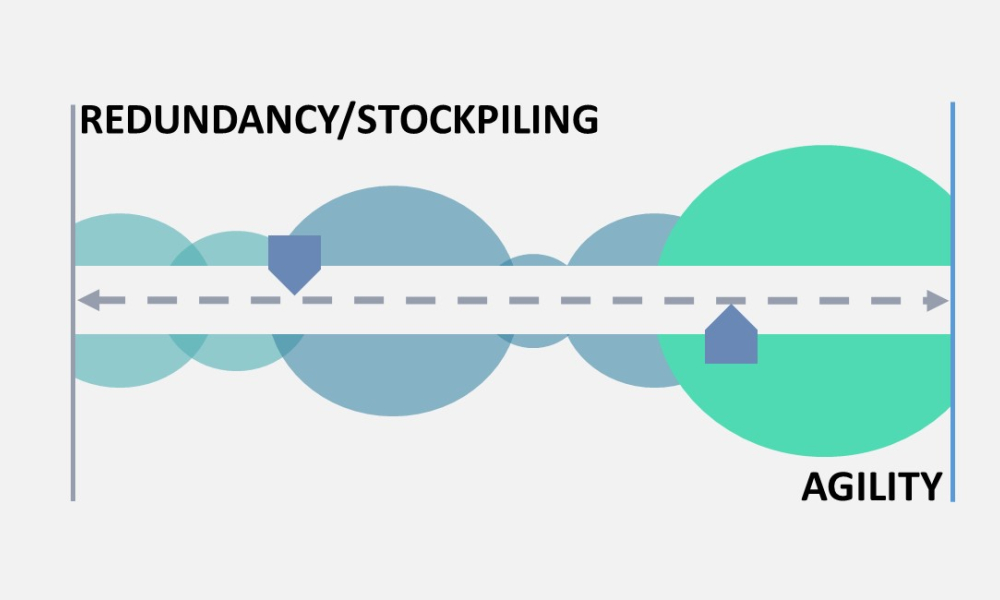
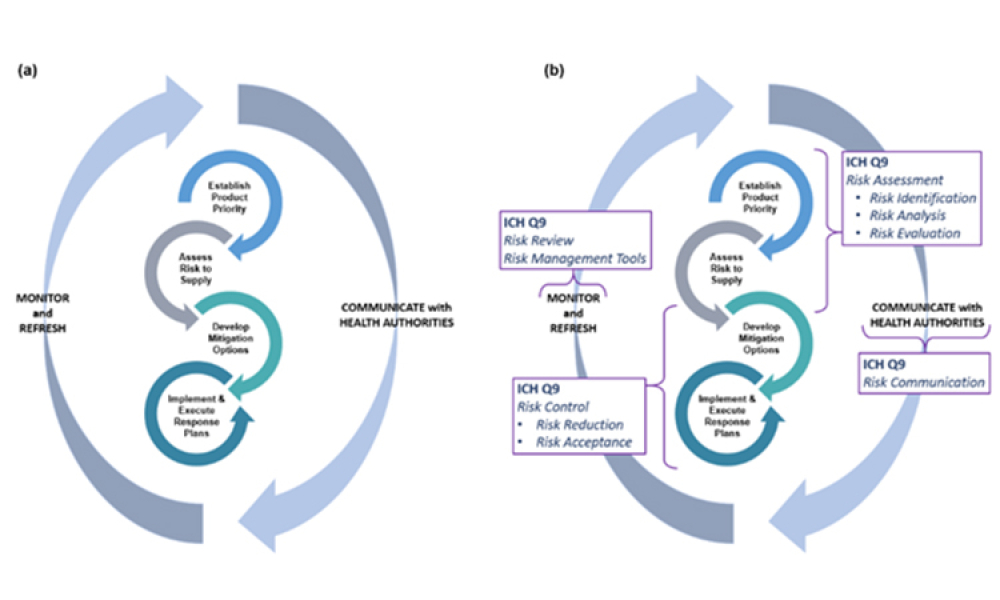
The regulation of drug shortage prevention has rapidly changed over the past several years due to large-scale, highly visible events, such as the COVID-19 pandemic, hurricanes, and geopolitical issues. Efforts to effectively address the complex and multifaceted issues contributing to drug shortages require close technical collaboration and clear communication between the pharmaceutical...