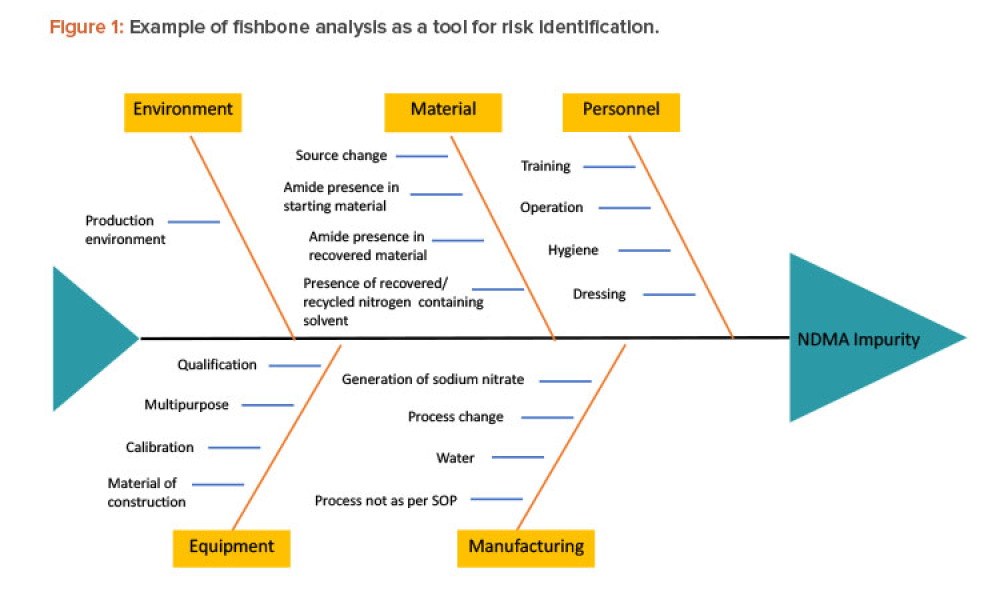
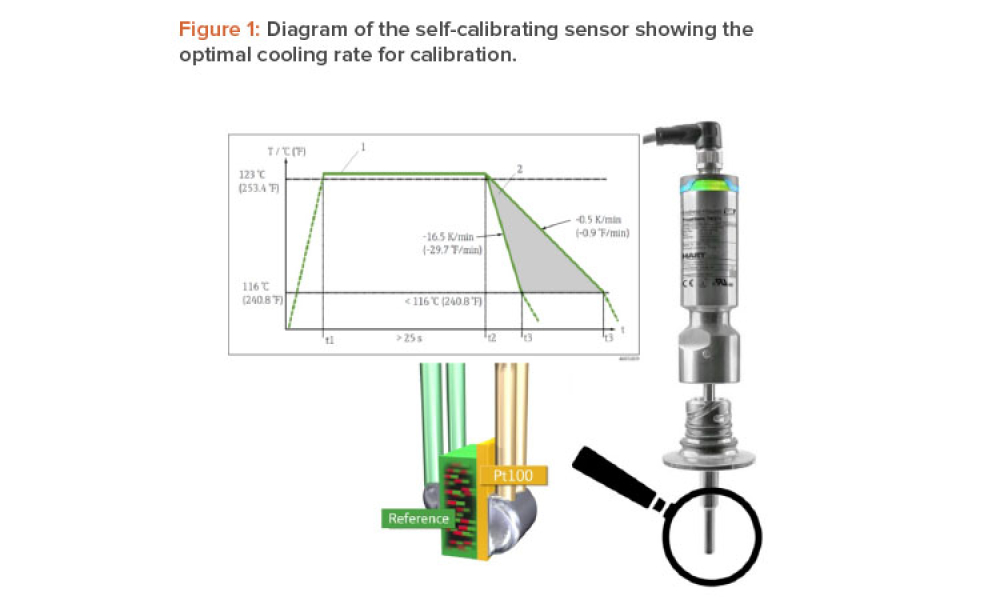
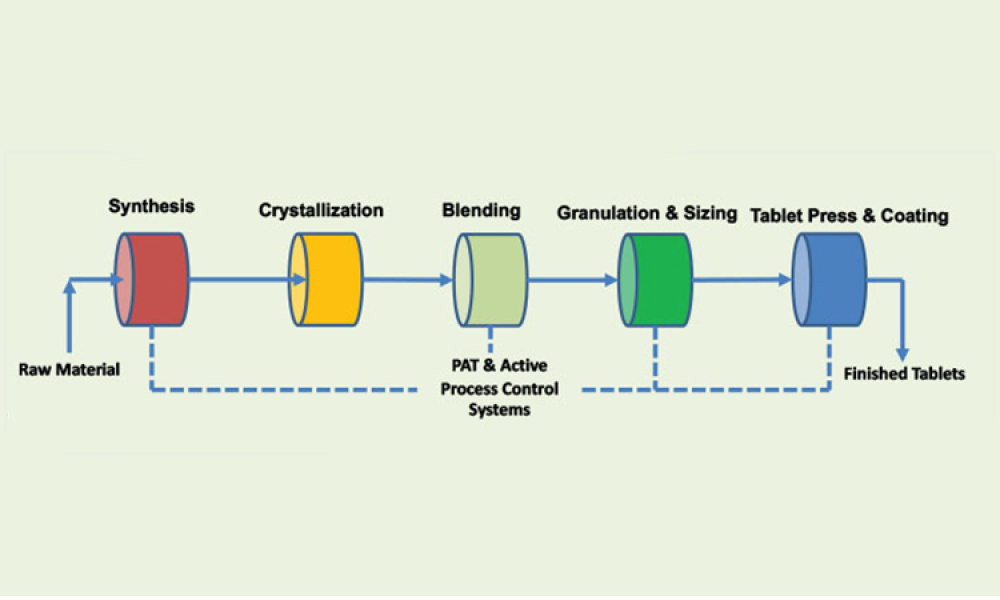
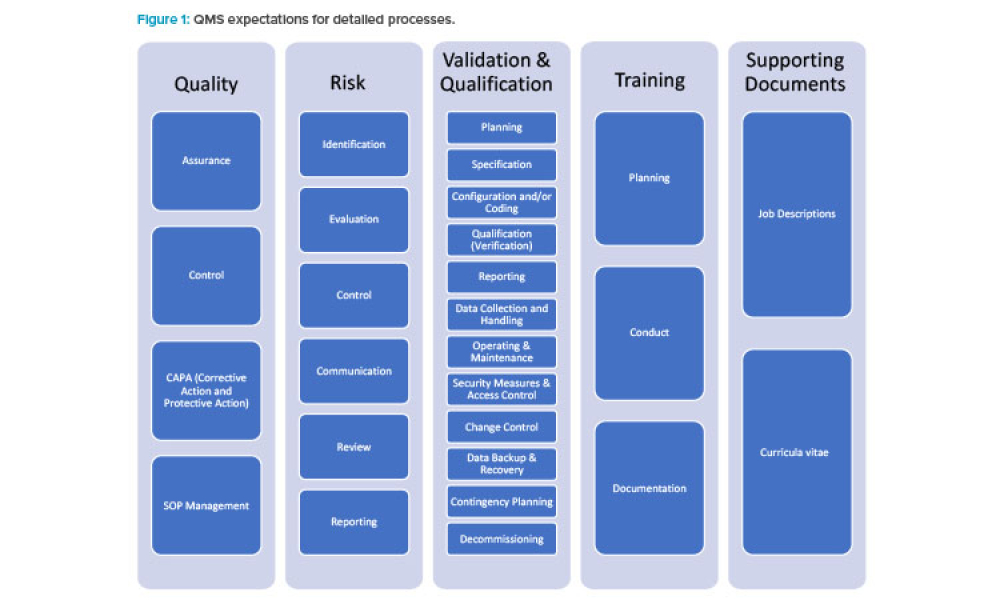
When you’re in the pharmaceutical industry, you’re going to be audited — are you ready? Authored by Kneat’s audit expert, Joe Azzarella, Audit Preparedness: Using Digital Validation to Be Ready for Anything, explains what you should expect before, during, and after an inspection and what you can be doing to make sure you pass with flying colors.
The ISPE Commissioning & Qualification (C&Q) Community of Practice (CoP) conducted a survey in 2023 on the adoption of integrated C&Q, specifically on the use of paperless digital systems for planning, executing, and reporting C&Q activities. The survey revealed that 74% of respondents planned to use Digital Validation Tools (DVTs) for C&Q by 2024.