Applicability of Vaporized Hydrogen Peroxide for Contamination Control of Lyophilized Biohazards

Controlling contamination in environments where biological medicinal products are handled is of paramount importance to ensure the safety of personnel, sterility of drug products, and protection of the surrounding environment. The application of vaporized hydrogen peroxide (vH2O2) has emerged as a promising method for postproduction decontamination due to its ability to degrade biohazards into nontoxic byproducts.
The majority of biologics and advanced therapy medicinal products (ATMPs) are parenterals that must be filled aseptically to maintain structural integrity and therapeutic efficacy, because terminal sterilization is not possible due to the instability of the product. Therefore, filling is often performed in isolators to ensure drug product quality and safety. Due to their sensitivity, biologics are often freeze-dried to increase product stability and shelf life.
This study explores the efficacy of vH2O2 decontamination for lyophilized biologics and ATMPs, considering the expected challenges for lyophilized drug products. We conducted experiments to investigate the influence of lyophilization and formulation composition, by using pharmaceutically relevant matrices, on the efficacy of vH2O2 decontamination. Our study highlights the difficulties in decontaminating lyophilized drug products in different pharmaceutically relevant matrices and proposes an isolator design solution to mitigate these challenges.
Introduction
Biologics and ATMPs—which include proteins, peptides, antibodies, vaccines, active viruses, and viral vectors, constitute a significant element of approved medical treatments and ongoing clinical trials. Until now, most biologic drug products were designed and destined for the parenteral route of administration. In most cases, aseptic filling is required to retain their structural integrity and therapeutic efficacy, instead of terminal sterilization, which would lead to severe degradation of the sensitive molecules.1
Handling genetically modified microorganism-based biologics and ATMPs can pose biohazardous risks to humans and the environment, and requires handling under the appropriate biosafety level. Contamination control in environments where these potentially hazardous biologics and ATMPs are handled is crucial to prevent accidental releases of the microorganisms to the environment and ensure product quality. Furthermore, cross-contamination is a central concern in filling suites for biologics and ATMPs, especially given the smaller batch sizes and diverse products being processed. Within the aseptic filling process, isolators play a vital role in maintaining product integrity and ensuring personnel and environmental safety.2
The use of vH2O2 is a well-established approach for decontamination of surfaces due to its ability to destroy a wide range of microorganisms, including viruses and spores. 3, 4 Therefore, it is also used for postproduction decontamination of isolators, restricted access barrier systems (RABS), and freeze dryers to eliminate any potential sources of contamination that may have arisen during the manufacturing process.5 To encourage ethylene oxide sterilization alternatives, the US Food and Drug Administration (FDA) recently categorized vH2O2 as a category A method of sterilization for medical devices.6 In Germany, the Association for Applied Hygiene (VAH) approved vH2O2 as a disinfectant, but exclusively for the decontamination of visually clean surfaces.7
All biopharmaceuticals are susceptible to degradation due to their macromolecular complexity and sensitivity to environmental conditions (e.g., temperature, pH, light). Particularly viruses, which can be used as vectors in ATMPs as well as live vaccines, pose biohazard risk (biosafety level ≥ 1).8, 9, 10 Moreover, viruses are susceptible to water-mediated destabilization and degradation pathways above 8°C. Typically removal of bulk water significantly increases the stability of viruses. Thus, most of the virus-based vaccines are freeze-dried drug products.11
For product and operator protection, the entire filling and lyophilization process of (genetically modified) microorganism-based biologics and ATMPs must be executed under aseptic conditions. During these processes, there are multiple critical steps identified that could cause a contamination of he vial surfaces and isolator chamber with free-dried, biohazardous drug product material.
This article presents the results of experimental studies conducted to investigate the vH2O2 decontamination efficacy of lyophilized Geobacillus stearothermophilus spores in the dependence of different pharmaceutically relevant matrices. Based on these results, it can be concluded whether decontamination with vH2O2 is applicable as postproduction decontamination of biohazardous, lyophilized biologicals and ATMPs, or whether additional requirements need to be placed on the design of the barrier system.
Experimental Design and Results
For the semiquantitative determination of the decontamination efficacy of a vaporized 35% aqueous hydrogen peroxide solution, 2 × 106 Geobacillus stearothermophilus spores, which represents a respected bioindicator (BI) for the process validation of vH2O2 decontamination, were diluted in a 300 µL matrix. In total, four different pharmaceutically relevant aqueous matrices were used (see Table 1) to investigate the impact of the formulation composition and resulting lyophilized cake on the vH2O2 decontamination efficacy.
To assess the influence of the dry cake on the vH2O2 decontamination, efficacy samples were either freeze-dried in a pilot-scale freeze-dryer (Epsilon 2-4 LSCplus, Martin Christ Gefriertrocknungsanlagen GmbH, Osterode, Germany) (see Table 2 and Table 3 for freeze-drying protocol), or heat-dried in a drying cabinet (UFP 550, Memmert GmbH + Co. KG, Schwabach, Germany) at 60°C for 24 hours.
After successful lyophilization, the different formulations resulted in dry cakes with structural and physical heterogeneity. Spores diluted in water only (control) did not form a visible cake, due to the lack of bulking agents. Based on the amorphous disaccharide sucrose content in FB1 and FB3, formulations 1 and 3 lead to amorphous lyophilized cakes after drying. In contrast, the ratio of four parts crystalline mannitol and one part amorphous sucrose in FB2 is expected to form a crystalline lyophilized cake.12
| # | Matrix |
|---|---|
| Control | Water |
| FB1 | 5% sucrose |
| FB2 | 20 mM histidine-HCl, pH 6.5, 4% mannitol, |
| FB3 | 1% sucrose |
| 20 mM histidine-HCl, pH 6.5, 1 mM magnesium chloride (MgCl2), 35 mM sodium chloride (NaCl), 7% sucrose, polysorbate 80 (0.02%) |
| Step | Shelf Temperature [°C] | Time [hh:mm] | Vacuum [mbar] | Safety Pressure [mbar] |
|---|---|---|---|---|
| Loading | 20 | - | - | - |
| Freezing | -45 | 01:05 | - | - |
| Freezing | -45 | 02:00 | - | - |
| Main Drying | -45 | 00:01 | 0.180 | - |
| Main Drying | -23 | 00:20 | 0.180 | 0.77 |
| Main Drying | -23 | 26:00 | 0.180 | 0.77 |
| Main Drying | -23 | 00:01 | 0.05 | - |
| Final Drying | 20 | 00:20 | 0.05 | - |
| Final Drying | 20 | 07:30 | 0.05 | - |
| Step | Shelf Temperature [°C] | Time [hh:mm] | Vacuum [mbar] | Safety Pressure [mbar] |
|---|---|---|---|---|
| Loading | 20 | - | - | - |
| Freezing | 5 | 00:15 | - | - |
| Freezing | 5 | 01:00 | - | - |
| Freezing | -40 | 01:30 | - | - |
| Freezing | -40 | 04:00 | - | - |
| Main Drying | -40 | 00:06 | 0.133 | off |
| Main Drying | -10 | 01:30 | 0.133 | 0.26 |
| Main Drying | -10 | 20:00 | 0.133 | 0.26 |
| Final Drying | 20 | 02:30 | 0.133 | 0.26 |
| Final Drying | 20 | 06:00 | 0.133 | 0.26 |
Heat-dried samples did obviously not result in a lyophilized cake, but formed a thin layer at the bottom of the vials because of collapse when drying is performed at high temperature. Heat-dried and freeze-dried samples were exposed to vH2O2 gasing cycle for up to 60 minutes. Photos of the dried units are depicted in Figures 1A–1H. The amplification of Geobacillus stearothermophilus was evaluated after an incubation period of seven days (see Figure 1I).
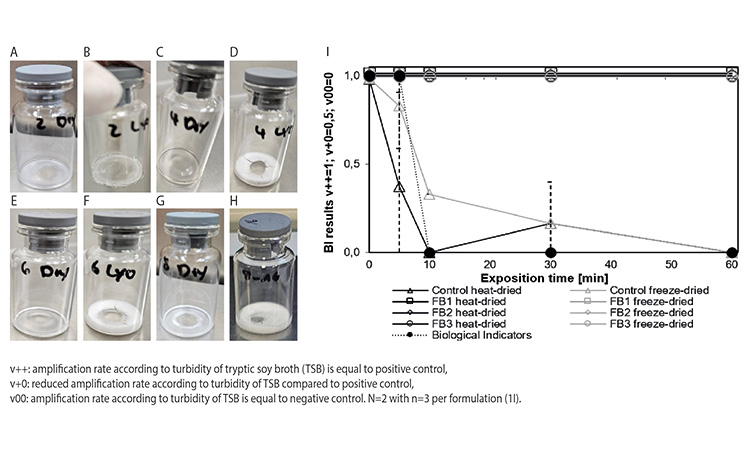
In the proof-of-concept pre-test, Geobacillus stearothermophilus spores diluted in water were tested to determine if they could still replicate after undergoing heat-drying, respectively, the lyophilization. In addition, it was examined if vH2O2 could distribute within the 6R vials in sufficient quantity and in less than 10 minutes to inactivate the Geobacillus stearothermophilus spores. The usual gassing time for a chamber of this size is approximately 20 minutes for inactivation of BIs at all challenge locations and 5–10 minutes for inactivation of BIs at the location where the samples were actually placed in this experiment setting.
As a control, the commercial biological indicator carriers (Mesa Labs, 1.9106 spores per carrier) were added in 6R vials and underwent the same vH2O2 gassing cycle. After the gassing cycle, the vials were filled with 6-millimeter (mL) TSB growth media and incubated at 57.5°C for seven days. Vials without any BI, filled with TSB only, served as negative control. To evaluate the amplification of Geobacillus stearothermophilus in TSB, the turbidity of the samples was compared with the turbidity of the negative control (TSB only) and the positive control (non- vH2O2 treated BI in TSB).
The first evaluation of growth was performed after 16 hours, and the final evaluation after 7 days. The non- vH2O2 treated lyophilized and heat-dried bacteria exhibited a similar growth kinetic as the commercial bioindicator (data not shown), thus neither the heat-drying nor the freeze-drying of the spores in water affected the viability of the spores. Furthermore, it was demonstrated that the commercial biological indicators in vials, heat-dried spores, and freeze-dried spores exhibit similar vH2O2 inactivation time in this pre-test (data not shown). These findings were essential to perform the next experiments.
Next, the impact of the matrix on the vH2O2 inactivation of freeze-dried and heat-dried Geobacillus stearothermophilus samples was evaluated. Therefore, the freeze-dried and heat-dried samples were subjected to the vH2O2 cycle for 5, 10, 30, and 60 minutes. The biological indicators, the lyophilized spores and heat-dried spores in water, for which no solid cake was formed, were inactivated after 60 min vH2O2 gassing. In contrast, the Geo-bacillus stearothermophilus spores in the other matrices, embedded in the dried matrices, still exhibited bacterial growth, therefore were not inactivated after 60 minutes of vH2O2 treatment, regardless of heat-dried or freeze-dried (see Figure 1I).
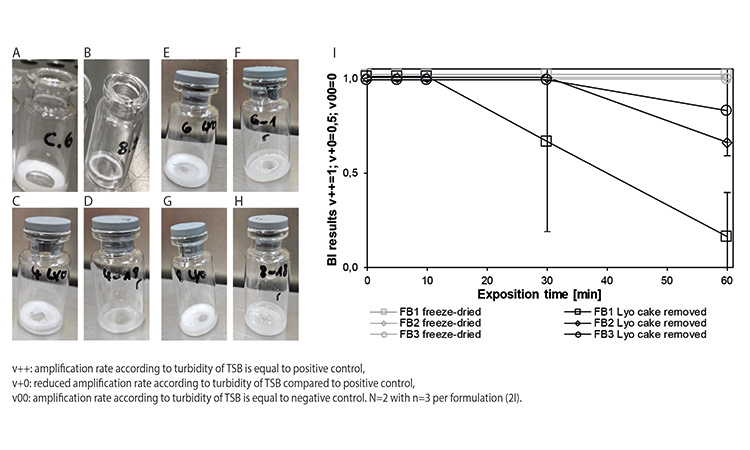
Subsequently, the effect of the lyophilized cake surface and thickness on the vH2O2 decontamination efficacy was evaluated using the FB1 formulation (pre-study “lyo cake”). Therefore, an intact FB1 lyophilized cake was crushed and mostly removed from the vial, leaving only a thin dust layer of the Geobacillus stearothermophilus containing lyophilized cake on the inner surface of the glass vial. In contrast to the intact FB1 lyophilized cake, which was partially dissolved after the vH2O2 gassing cycle (Figures 2A and 2B) and where the spores could not be inactivated even after 60 minutes, the thin lyoph-ilized cake dust layer containing Geobacillus stearothermophilus exhibited a reduced bacterial growth rate after a 60-minute vH2O2 gassing cycle (data not shown).
Integrated return air fi lters located directly at the main isolator chamber pose a solution to contain possible powder within the chamber and to make it accessible for cleaning.
Based on the pre-study “lyo cake,” lyophilized cakes of formulations FB1, FB2, and FB3 were removed and the weight of the lyophilized cake dust layer remains was determined. The FB1 lyophilized cake could be removed very efficiently, resulting in a very thin lyophilized cake dust layer and small lyophilized cake crumbles (see Figures 2C and 2D). For FB2 (see Figures 2E and 2F) and FB3 (see Figures 2H and 2G), removing the lyophilized cake was more difficult, leaving behind a thicker dust layer with larger lyophilized cake crumbles. The medium amount of removed material was 90% for FB1, 31% for FB2, and 20% for FB3.
The vials with the different lyophilized cake dust layers underwent the same vH2O2 gassing cycle as before. After a 60-minute vH2O2 gassing cycle, the heat-dried formulations (data not shown) and the intact lyophilized cakes exhibited an unreduced bacterial growth rate compared to the positive control. Also, the bacterial growth rate of the FB2 and FB3 lyophilized cake dust layers were almost unaffected and the bacterial growth rate of the FB1 lyophilized cake dust layer was only marginally reduced, even after a 60-minute vH2O2 exposure time (see Figure 2I.)
Discussion
The use of vH2O2 is a well-established, safe, and cost-effective method for room and full-system decontamination of visually clean surfaces after the handling of biohazardous products (postproduction cycle). This helps protect the operator, prevent the release of biohazardous products in the environment, and reduce the risk for cross-contamination within a production facility/filling line. Using vH2O2 as a postproduction decontamination method has advantages over formaldehyde. This is because vH2O2 can be removed from the system with catalysts where it is degraded to oxygen and water, leaving no liquid waste or cancerogenic substance for disposal.
Because the vH2O2 bio-decontamination is exclusively approved for the disinfection of visually clean surfaces, this work addressed the question of whether vH2O2 might be a suitable disinfectant to decontaminate surfaces contaminated with lyophilized hazardous biologicals or ATMPs. In addition, the impact of the matrix as well as the size of the lyophilized cake crumbles on the vH2O2 efficacy was investigated by using Geobacillus stearothermophilus as a surrogate.
The experiments demonstrated that each tested pharmaceutical relevant matrix as well as the thickness and grain size of the lyophilized BI residues have a huge impact on the vH2O2 decontamination efficacy (see Figure 1I). The intact lyophilized cakes of formulation FB1, FB2, and FB3 prevented the vH2O2 inactivation of the lyophilized Geobacillus stearothermophilus within the tested exposure time of 60 minutes. This might be due to a combination of following effects:
- Limited penetration capability: vH2O2 is known to have a limited penetration capability, therefore the German competent authority among others approves vH2O2 explicitly for the surface disinfection of visually clean surfaces.13
- Dissolution of the lyophilized cake: pre-study “lyo cake” (see Figures 2A and 2B) showed that the cake dissolves during the vH2O2 gassing process in liquid vH2O2, condensed water, or probably a mixture of liquid vH2O2 and condensed water. Therefore, the spores are exposed to liquid vH2O2 instead of vH2O2. Research has demonstrated that vH2O2 in its vaporized form is a stronger oxidizing agent than in its liquid form.14 In addition, the concentration of vH2O2 in the gas phase is higher than in the liquid phase.15
Notably, the bacteria embedded in the lyophilized cake dust layers, after removal of the lyophilized cake itself, could not be inactivated, even after 60-minute vH2O2 exposure time, because the bacterial growth rates in FB2 and FB3 were nearly unaffected and were only marginal reduced in FB1.
In order to classify the results of the experiments correctly, it should be noted that the well-established bioindicator organism Geobacillus stearothermophilus presents a great challenge in the realm of deactivation. It is expected that inactivation times for hazardous biologicals and ATMPs will be extended in a similar proportion or to a similar extent depending on the formulation.
The experiments demonstrate that bacteria embedded in a dried matrix are inaccessible for the vH2O2 penetration, even when it is only a thin lyophilized cake dust layer or small lyophilized cake crumble. Further, the results point toward that the formulation composition (and with it the cake structure and morphology) play only a minor role in counteracting the vH2O2 decontamination as FB1, FB2, and FB3, which represent pharmaceutically relevant matrices, all posed a similar challenge to successful decontamination (see Figure 2I). When these findings are traced back to the actual fill/finish process, it can be concluded that lyophilized material must be prevented from accumulating anywhere in the system if vH2O2 is used for post-production bio-decontamination.
To meet the challenge posed by accumulated lyophilized products inside the isolator chamber, a specialized isolator design is proposed (see Figure 3). This design aims to make the space where lyophilized product is contained clear and accessible. Especially, an accumulation in poorly accessible return air ducts or plenum areas should be prevented. Therefore, the incorporation of return air filters, which are located directly on the isolator chamber, is suggested.
These filters capture product powder that may occur inside the isolator chamber and prevent the agglomeration of freeze-dried biohazards within the isolator return air ducts and plenum. Automatic return air filters are preventing biohazardous powder entering return air ducts and plenum areas. Filters can be changed without exposure (safe change).
The air lock filters are decontaminated during gassing with vH2O2 , as the vapor can penetrate through the filter (data not shown). When exchanging the filters, safe change principles are applied, thus larger lyophilized cake crumbles that are not inactivated by vH2O2 do not pose a risk to the operator or the environment. Furthermore, the isolator design is prepared for wash-in-place integration. If based on the product, this method is chosen for decontamination after spillage of lyophilized drug product.
Conclusion
We found that vH2O2 is an effective method with wide spectrum activity for controlling contamination with biohazards. When lyophilized hazardous biologicals and ATMPs are present in the system, accumulation of the lyophilized powder poses a risk to vH2O2 postproduction decontamination. Therefore, during the design of the barrier system, process and safety requirements should be considered. Integrated return air filters located directly at the main isolator chamber pose a solution to contain possible powder within the chamber and to make it accessible for cleaning. The combination of post-production decontamination and return air filter creates a robust contamination control solution for production of lyophilized hazardous biologics and ATMPs.
Acknowledgments
Ann-Catherine Roth, Katharina Westerhoff, and Dennis Kreft for execution of experiments at Franz Ziel GmbH
Holger Kranenburg, PhD, for pre-studies at Franz Ziel GmbH
Mónica Rosa, PhD, and Brecht Vanbillemont, PhD, for discussions about lyophilization matrices at Coriolis Pharma Research GmbH



