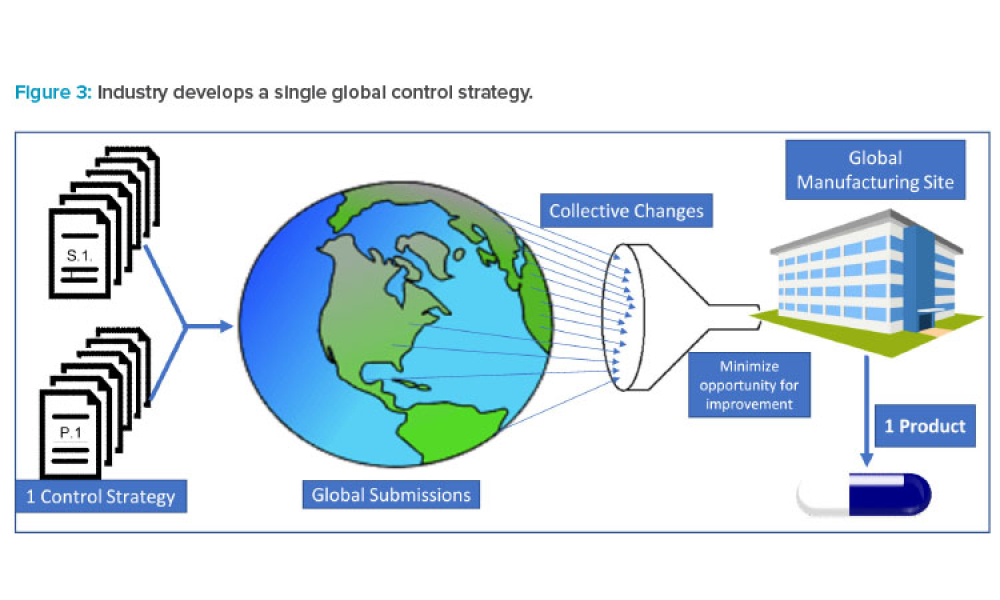
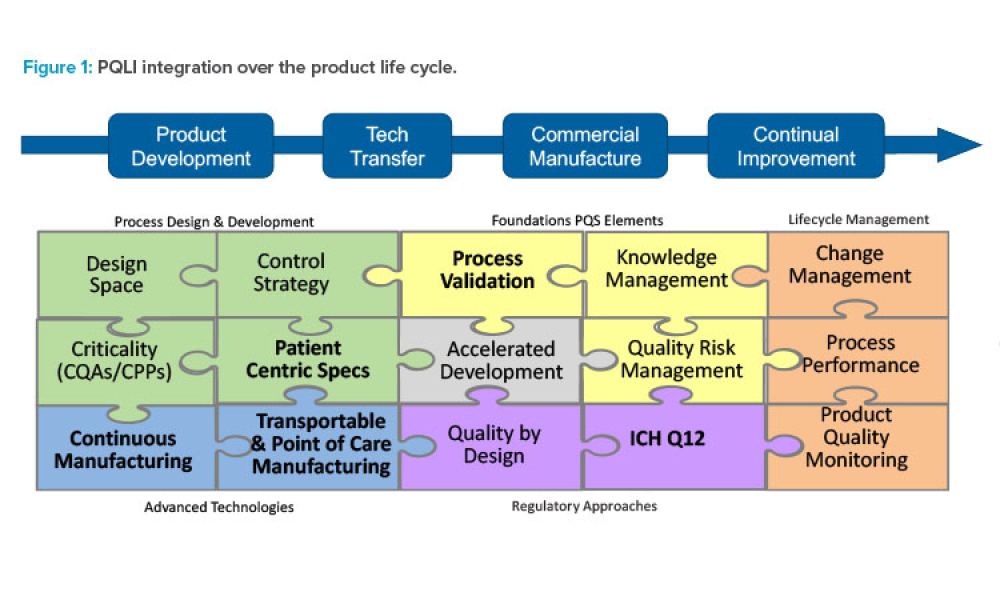
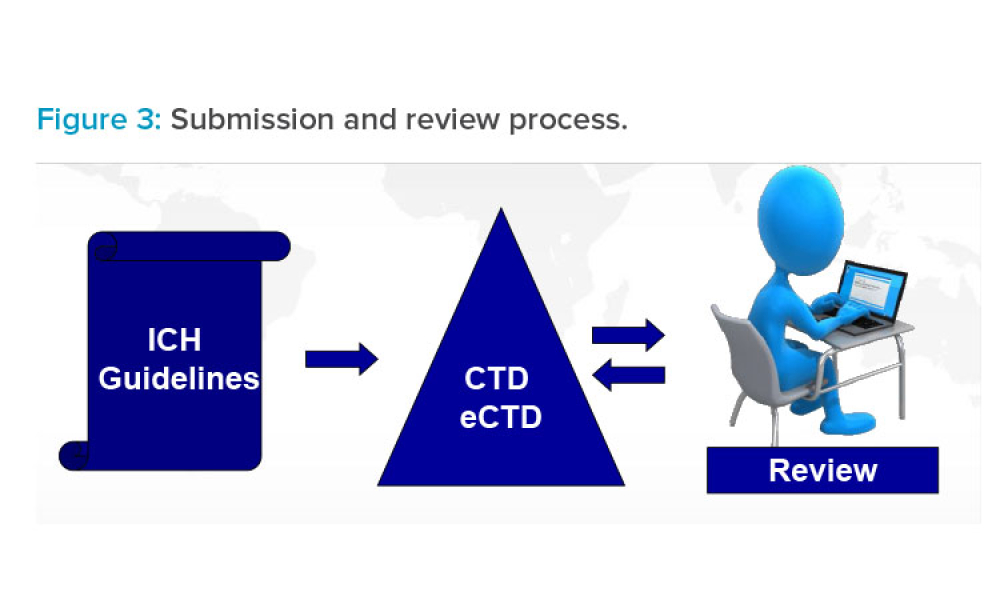
Validation professionals are under pressure — from rising workloads to new regulatory demands. Backed by four years of industry data, this year’s State of Validation report — authored by leading life sciences market research Jonathan Kay — reveals how organizations are tackling challenges like audit readiness, resourcing constraints, and the shift to digital systems. Discover benchmarks, best...
ISPE’s Q12 Implementation Team continued its series of training events with a well-attended course for Singapore’s Health Sciences Authority (HSA) in November.