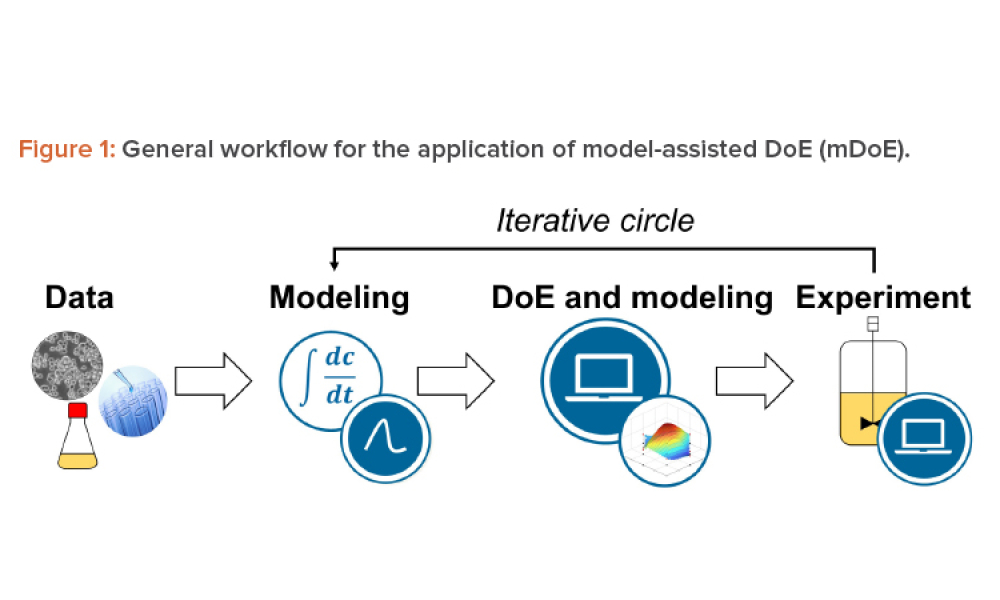
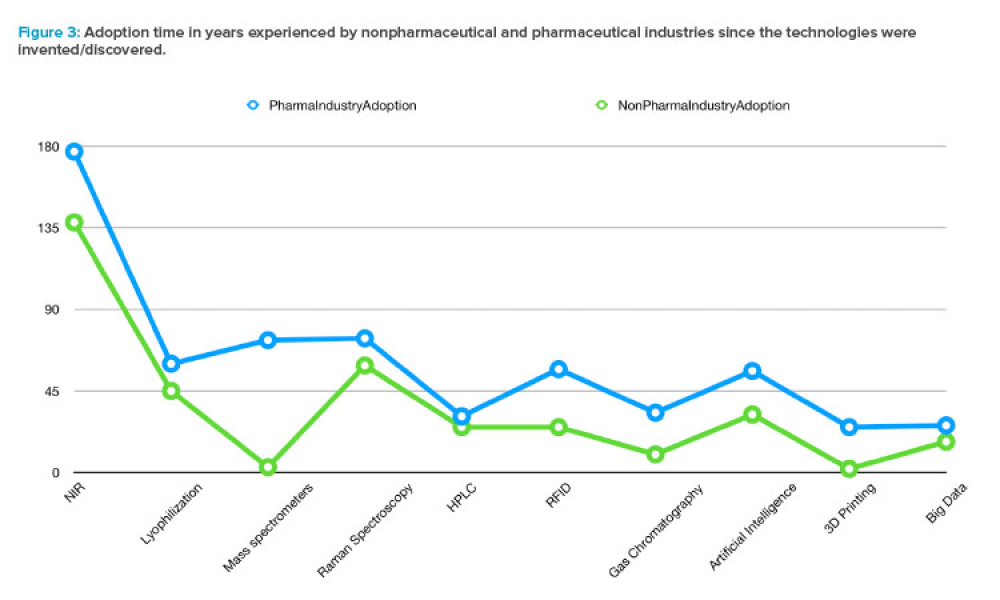
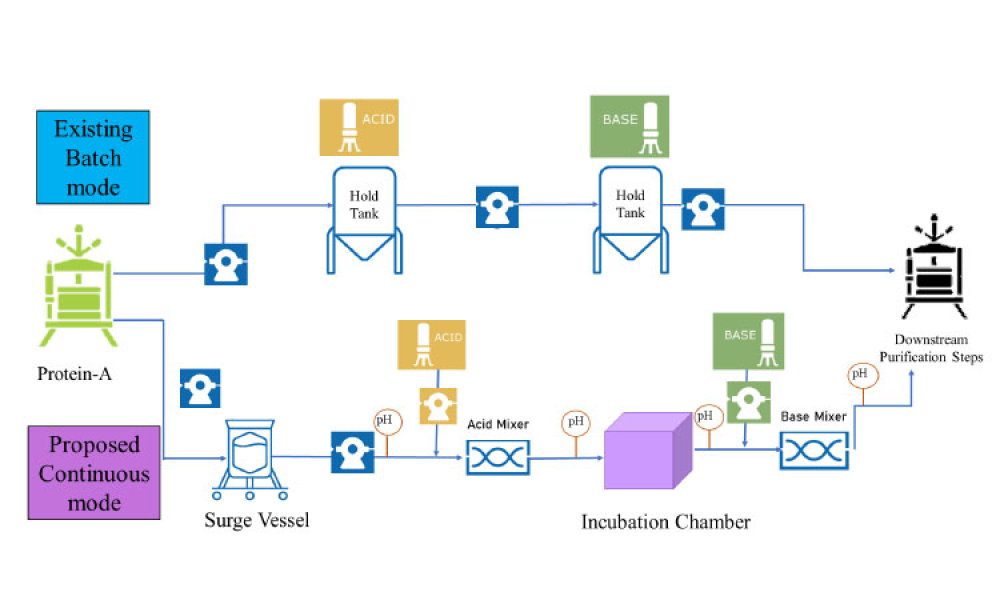
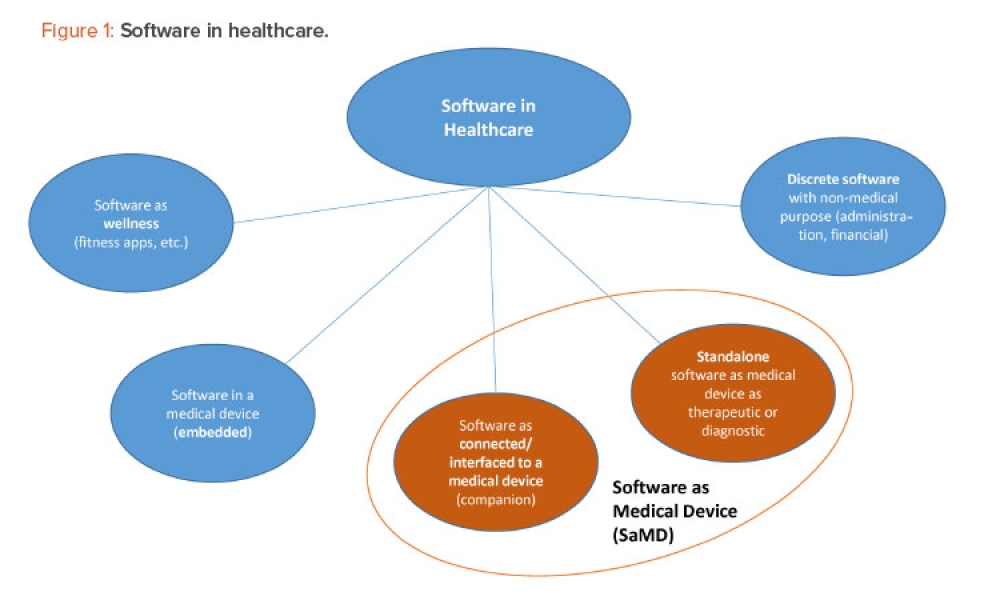
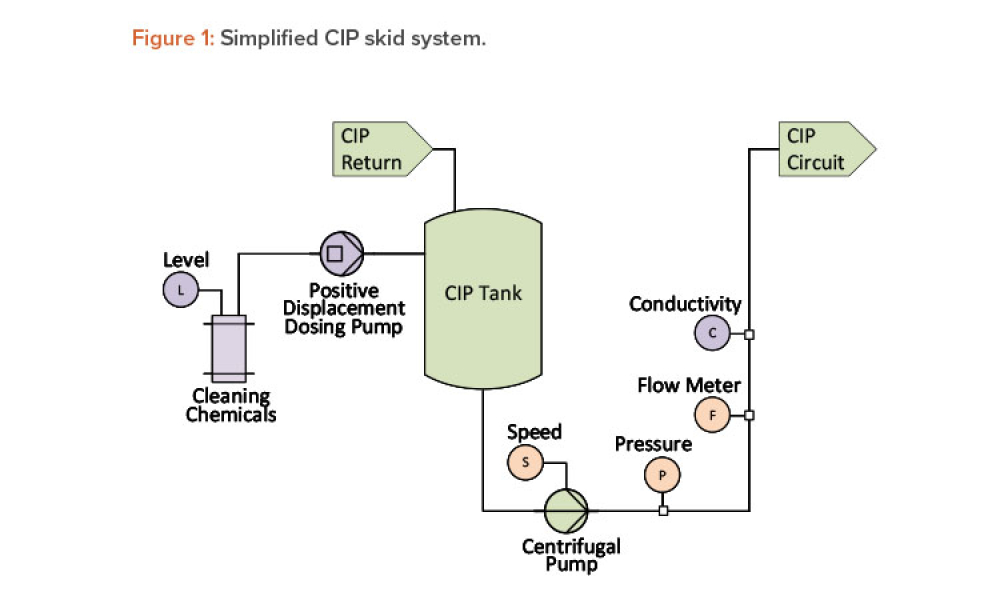
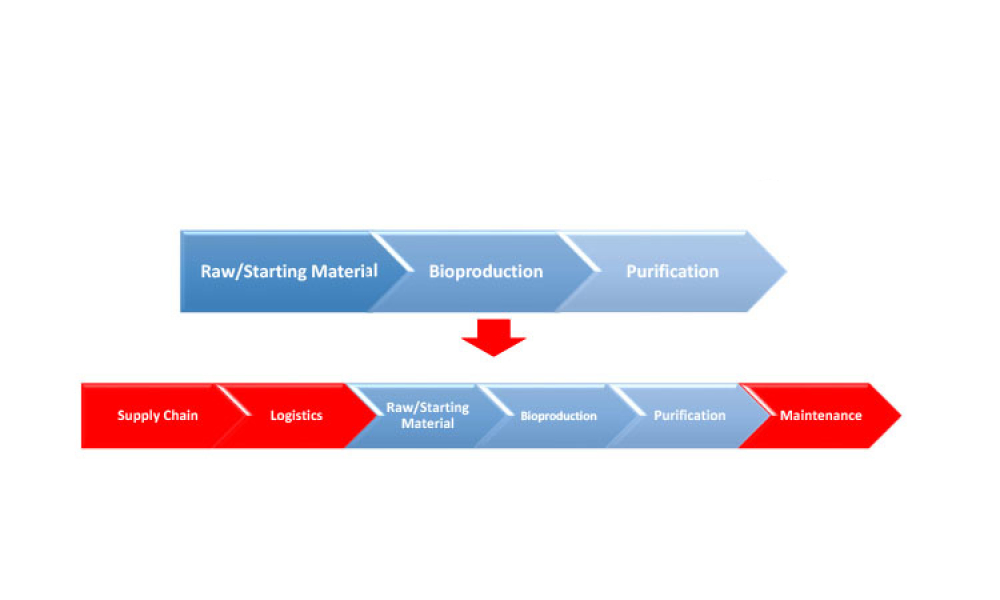
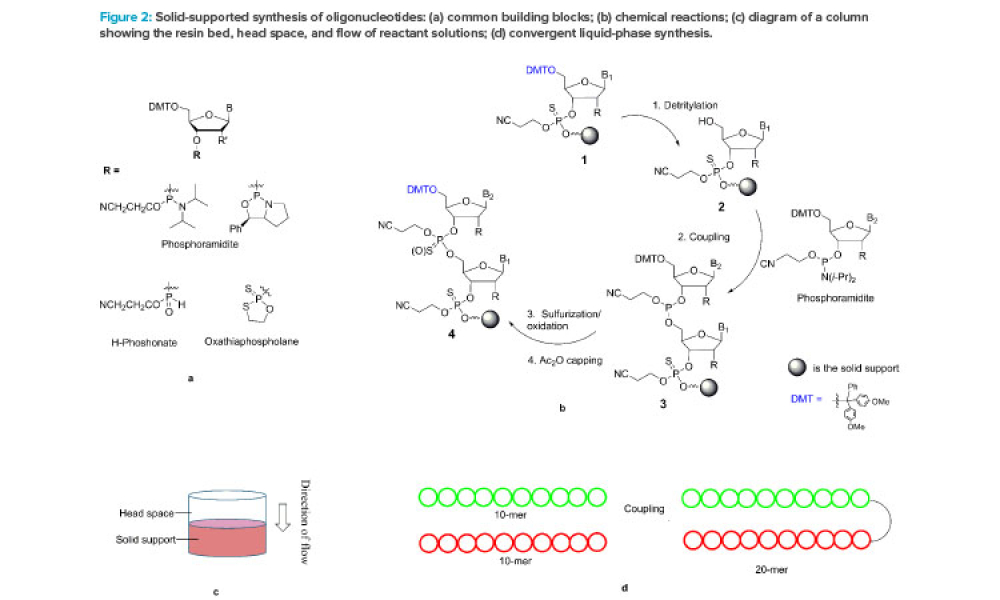
Cell therapies, especially autologous chimeric antigen receptor T cell (CAR T cell) treatments, are transforming personalized medicine, bringing new hope to patients with conditions once thought untreatable. However, the manufacturing processes for these therapies remain predominantly manual, presenting significant challenges in scalability, consistency, and making these treatments more widely...
Advanced therapy medicinal products (ATMPs), which include cell and gene therapy (C>) products, frequently require handling steps between quality control release and patient administration. These steps take place directly at the point of care and are especially critical for C>s with limited shelf life after preparation.