Indian Pharmaceutical Industry: Creating Global Impact

India’s pharmaceutical sector is not only a cornerstone of its national economy, but it’s also a pivotal player on a global stage. As of 2023, India ranked as the third-largest producer of drugs and pharmaceuticals by volume, with a 20% global share in the export of generic drugs. Currently valued at US$50 billion, the country has an ambitious goal for the sector: to grow its value to US$450 billion by 2047.
The country’s strength in manufacturing high-quality generic drugs has made it a reliable supplier to over 200 countries and regions, including highly regulated markets of the United States, United Kingdom, and European Union. This success is driven by a skilled workforce, cost-efficient production methods, and a regulatory framework that supports business growth. India’s commitment to research and development, along with its emphasis on high-quality generic medications, has reinforced its status in the global pharmaceutical market.
The Federation of Indian Chambers of Commerce and Industry projects the total market size of the Indian pharmaceutical Industry will reach US$120 billion by 2030 11 , fueled by a heightened focus on innovation and growing export opportunities. With government initiatives aimed at enhancing local manufacturing and attracting foreign investment, India is set to emerge as a global leader in pharmaceutical production. This will allow substantial contributions to both the economy and the healthcare system.
India: Firmly Established in the Generic Drug Space
Now, as a key global player in the pharmaceutical industry with a vital role in producing and distributing medications worldwide, India has one of the world’s fastest-growing economies and is on track for becoming one of the world’s top three economic powers in the next decade, according to the India Brand Equity Foundation. The development of a robust pharmaceutical supply chain network with manufacturing facilities has been a key factor in fueling the Indian economy.
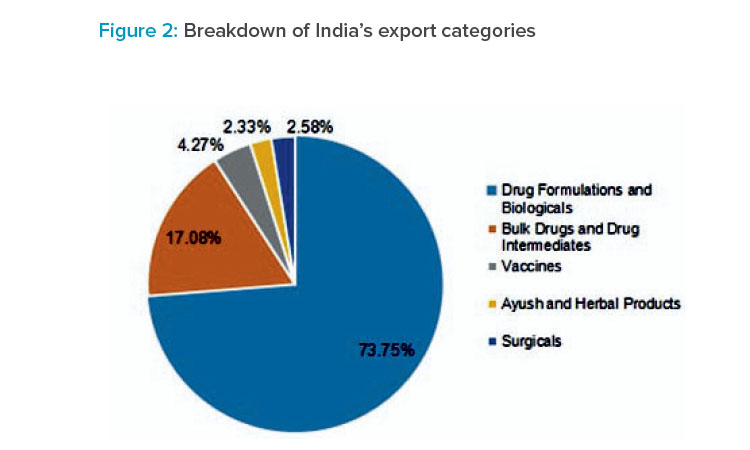
As of 2023, India ranked as the third-largest producer of drugs and pharmaceuticals by volume, with a 20% share in the export of generic drugs 1 , 5.71% of the global share of all pharmaceutical exports 1 , and a 60% share in the supply of low-cost vaccines 15 , according to the Indian Pharmaceutical Alliance (IPA).
The sector has the potential to exceed US$120 billion by 2030 11 . Leading Indian pharmaceutical companies have strategically positioned themselves as strong contenders in the expanding generic market in North America. Additionally, many Indian companies have firmly established themselves in the specialty medicines market. For example, the oncology drug market in India is expected to reach US$1.98 billion this year 17 .

Indian pharmaceutical companies have achieved global recognition through competitive pricing and their ability to manufacture cost-effective generic alternatives, with many benefiting from economic factors that support the industry, including competitive land rates, low resource expenses like water and electricity, and affordable machinery costs.
Between FY 2018 and FY 2023, the Indian pharmaceutical industry saw steady growth, with a compound annual growth rate (CAGR) of 6%–8%, primarily driven by an 8% increase in exports and a 6% rise in the domestic market 6 . This sector has attracted significant foreign direct investment (FDI), crossing the US$20 billion mark in September 2022, with FDI inflows increasing fourfold over five years 12 .
On 17 December 2024, the Ministry of Chemicals and Fertilizers in India announced that for FY 2023–2024 (which closed 31 March 31 2024), the pharmaceutical market was valued at US$50 billion 6 with domestic consumption accounting for US$23.5 billion and drug and pharmaceutical ex-ports US$26.5 billion 15 , which compares with US$15 billion in exports for FY 2013–2014 14 (see Figure 1). The sector is projected to achieve a turnover of US$130 billion by 2030 6 .
India’s Domestic Pharmaceutical Market
India also boasts a strong domestic pharmaceutical industry. This industry plays a crucial role in providing medications to the Indian population and other countries. With a wide-reaching network of drug companies and manufacturing facilities, India’s pharmaceutical sector significantly contributes to the nation’s economy and healthcare system. The country is home to over 3,000 drug companies and approximately 10,500 manufacturing units. These include 7 :
- More than 2,000 units hold World Health Organization (WHO) GMP approval
- 253 plants are approved by the European Directorate of Quality Medicines
- 1,105 units possess Europe’s Certificate of Suitability (CEP)
- Over 950 units comply with the Therapeutic Goods Administration guidelines
- 500 are approved by the US Food and Drug Administration (the highest number outside of the US)
INDIA’S DIFFERENTIATORS
Indian pharmaceutical companies have achieved global recognition through competitive pricing and their ability to manufacture cost-effective generic alternatives, with many benefiting from economic factors that support the industry, including competitive land rates, low resource expenses like water and electricity, and affordable machinery costs. Notably, India’s drug manufacturers integrate various components, such as intermediates, Active pharmaceutical ingredients (APIs), and formulation companies, while adhering to international safety and quality standards. The contract manufacturing sector plays a significant role in positioning India as a top global player by production volume.
India is often referred to as the “Pharmacy of the World.” Indian pharmaceutical sector supplies over 50% of global demand for various vaccines, 40% of generic demand in the United States and 25% of all medicine in the United Kingdom. The domestic pharmaceutical industry includes a network of 3,000 drug companies and 10,500 manufacturing units,” per the India Brand Equity Foundation (IBEF) 6 .
The industry offers a wide range of 60,000 different generic brands across 60 therapeutic categories, including generic drugs, over-the-counter (OTC) medicines, APIs/bulk drugs, vaccines, contract research and manufacturing, biosimilars, and biologics 2, 8 .
There are a number of noteworthy pharmaceutical companies listed on the National Stock Exchange (NSE) in India, including Sun Pharma Industries Ltd., Divi’s Laboratories Ltd., Cipla, Mankind Pharma, Dr. Reddy’s Laboratories, Zydus Lifesciences Ltd., Torrent Pharmaceuticals Ltd., Aurobindo Pharma Ltd., and Alkem Laboratories Ltd. 16 . India also excels in the production of APIs, with the Confederation of Indian Industry ranking the country’s API industry as the third-largest globally, contributing around 57% of APIs to the World Health Organization’s (WHO) prequalified list 6 .
The government’s initiatives to boost local manufacturing, particularly through the Production Linked Incentive (PLI), has gained momentum. Amid disruptions in the supply chain due to the COVID-19 pandemic, the government has introduced initiatives to support the production of bulk drugs and medical devices for domestic use and export. Under PLI, the government sanctioned funds for developing bulk drug parks to world-class infrastructure facilities that have common amenities like solvent recovery plants, distillation plants, power and steam units, and effluent treatment plants.
The Indian government has made modifications to the Pharmaceutical Technology Upgradation Assistance Scheme to improve quality upgradation for small and medium-sized enterprises 17 . This scheme, which is a part of the country’s “Strengthening Pharmaceuticals Industry” initiative, provides financial assistance for upgrading manufacturing facilities to meet international standards 18 . India’s focus on research and development aims to foster innovation and propel the nation toward becoming a global leader in pharmaceutical manufacturing.
The Indian pharmaceutical sector comprises of five key verticals: contract research and manufacturing services (CRAMS), APIs, formulations, biologics and biosimilars, and vaccines.
CRAMS
The contract development and manufacturing organization (CDMO) industry plays a vital role in the global drug development due to specialized expertise, cost efficiency, and ability to accelerate the drug development process. They also ensure regulatory compliance, offer scalable production, and allow pharmaceutical companies to focus on their core competencies, leading to faster and more efficient market entry for new drugs. The CDMO mar-ket in India is forecasted to reach US $44.6 billion by 2029 from an estimated size of US $22.5 billion in 2024 21 . In the US, nearly 50% of all new drug application (NDA) approvals involve contract-manufactured drug products, and over 50% of small-molecule NDA products involve contract-manufactured drug substances. The CDMO industry has enabled rapid expansion in the bio/pharmaceutical sector, with 80% of drugs for emerging companies and 60% for midsize companies being manufactured by CDMOs 19 .
CRAMS is undergoing substantial growth. It’s expected to grow by 6.2% annually from 2021 to 2026, reaching about $170 billion. Biologics-based CRAMS is set to grow even faster, at 11% annually from 2020 to 2026, due to an increasing number of medicines in development and the limited manufacturing expertise of respective companies. Small molecules also play a big role, with around 6,000 molecules currently in development 20 .
Many international pharmaceutical companies are using CRAMS and outsourcing research and clinical trials to developing nations as a strategy to address increasing costs and regulatory limitations in developed markets. This approach enables companies to concentrate on branding, tap into specialized product expertise, and mitigate risks while ensuring the delivery of top-notch medications to the market.
APIs
India’s APIs market was valued at US$11.8 billion in FY 2021 and is projected to grow at a CAGR of 12.24% from 2021 to 2027 22 . Numerous Indian companies contribute to API manufacturing, with this growth driven significantly by the expanding biopharmaceutical sector in the country.
Formulations Development
Globally recognized for high-quality generic medicines, India’s largest pharmaceutical companies are major players in manufacturing and exporting generics. They rank among the largest generic medicine producers worldwide.
Biologics and Biosimilars
The biologics and biosimilars 6 segments are gradually gaining traction in India. As of 2021, India held an 8% share of the global biopharmaceutical market 6 . The global biosimilars market is projected to reach US$1.3 trillion by 2032 23 , with Indian manufacturers producing US$500–$600 million in a US$12 billion market. India, could potentially, own 15%–20% of the global market 24 .
Vaccines
India is the world’s largest vaccine supplier, providing 62% of global demand. Currently, two-thirds of its vaccine production is exported. The Indian vaccine market was worth US$95 billion in 2021 and is projected to reach US$256 billion in 2030 9 .
Medical Device Manufacturing and Innovation
2023 marks a significant milestone for India’s medical technology industry with the introduction of the National Medical Devices Policy (NMDP) 25 , which aims to promote the Indian medical devices sector and position the country as a global leader in medical device manufacturing and innovation. The goal is to grow the sector to a point in which India owns 10%–12% of the global market over the next 25 years.
The NMDP outlines strategies for phased manufacturing of critical components and utilizes initiatives like the Public Procurement (Preference to Make in India) Order 2017 and the Aatm Nirbhar Bharat Abhiyan, or the Self-Reliant India Mission 26 to bolster domestic manufacturing. It charts a roadmap focusing on accessibility, affordability, quality, patient-centered care, preventive health, security, research, innovation, and skilled labor development.
The government is supporting medical device manufacturing in India through regulatory frameworks and industry-friendly policies. Initiatives like the production-linked incentive (PLI) scheme to encourage entrepreneurs to establish domestic facilities, possibly in collaboration with global partners. The integration of smart connected care, advancements in diagnostics and therapy, and improved clinical outcomes are shaping the market for medical devices and diagnostics. Growth areas include smart medical devices, digital therapeutics, AI-based solutions, predictive analytics, and wearable technology. India is one of the top 20 countries in demand for medical devices, but its local industry is still developing and relies heavily on imports of advanced medical technologies such as, cancer diagnostics, medical imaging tools, ultrasonic scans, high-value cardiology devices, hearing aids, and orthopedic implants.
“For sustainable development of the medical devices industry and safeguarding the interests of patients, India needs to actively reflect and work towards addressing the need for timely availability of crucial lifesaving medical technologies to citizens, without any discrimination, to deliver better patient outcomes, and also to keep our medical faculty and students updated on the latest medical technologies,” states the 2023 report, “Ensuring Equi-table Access to Critical Medical Technologies for Indian Citizens” by the Public Health Foundation of India and the Indian Medical Parliamentarians’ Forum (IMPF) ]32].
India is on the right path to capitalize on this opportune time, with key factors aligning to drive pharmaceutical manufacturing in the country. It has built a robust scientifi c and technological foundation through decades of extensive collaboration with pharmaceutical companies and research institutions worldwide.
By launching research and development initiatives and allowing 100% foreign direct investment (FDI), India aims to become a top manufacturer of medical devices in both local and global markets. Additionally, they aim to ensure their citizens have fair access to critical care medical devices 26 .
The report focuses on several key areas: analyzing current regulations for medical devices in India, assessing the demand for advanced medical devices, evaluating the procurement process, examining the cost-effectiveness of new medical devices, looking at how health technology assessment (HTA) is used globally, and reviewing new procurement methods like value-based procurement (VBP).
As India progresses toward becoming a global medical technology hub, fostering stronger alliances, leveraging technology effectively, and establishing clear policy frameworks are crucial. Initiatives such as developing more medical device parks, partnering with industry and academia to address skill gaps among healthcare professionals, and prioritizing mental health support for healthcare workers can further enhance India’s position as a medical device powerhouse. Success in seizing these opportunities will define India’s stature in the global medical technology industry.
Evolving Business Models and Opportunities
Pharmaceutical companies worldwide have numerous opportunities to enhance their investments in India. With a growing trend toward collaborative business approaches, Indian companies are expected to play a vital role as partners. Indian pharmaceutical companies are advancing toward more valuable endeavors. Overseas corporations are increasingly utilizing India’s growing research capabilities in addition to its manufacturing expertise. They collaborate through entering into licensing agreements, franchising, or joint ventures to tap into the Indian market. Among these, joint ventures are an increasingly popular option for companies aiming to take advantage of opportunities in India.
Foreign companies are seeking local partners to expand their presence in the country. Domestic partners offer valuable local expertise, knowledge, and networking capabilities. These advantages—coupled with cost-effective production, skilled labor, and expedited drug development—greatly benefit western pharmaceutical companies entering the Indian market.
Other companies are using local branches to establish their own sales and marketing operations, either through internal growth or acquisitions. GlaxoSmithKline (GSK) has 20 contract manufacturing organizations, and regional and sales hubs in India. In 2024, Novartis AG announced that it is conducting a review of Novartis India Limited, noting that it is “deeply committed to India with a footprint that has expanded significantly in recent years.”
Major Pharmaceutical Clusters in India
India is recognized as a global pharmaceutical hub, providing affordable and high-quality drugs that improve health worldwide. Many multinational companies have invested heavily in India and are growing their presence in different parts of the Indian pharmaceutical market. They are also expanding into smaller cities and rural areas to make healthcare more accessible to more people.
Regulators have supported the industry by introducing PLI schemes and specialized industrial zones (bulk parks) for APIs, which help Indian API manufacturers compete on an equal footing. These measures have also reduced the cost difference between India and China for APIs.
India’s pharmaceutical hubs present diverse investment opportunities in APIs, biosimilars, vaccines, nutraceuticals, and contract research. In March 2024, under the PLI Scheme, the Indian Ministry of Chemicals and Fertilizers inaugurated 27 Greenfield Bulk Drug Park projects and 13 Greenfield Manufacturing Plants for Medical Devices.
In 2022, the bulk drug park in Una, Himachal Pradesh, began construction. It is expected to attract investments of US$1.35 billion and create 20,000 jobs.
In southern India, at over 19,000 acres, Hyderabad Pharma City (HPC) in Telangana’s Ranga Reddy district (including Kandukur, Yacharam, and Kadthal Mandal) is reported to be the world’s largest integrated cluster for pharmaceutical industries in both research and manufacturing. Recognized as a National Investment and Manufacturing Zone by the Government of India, HPC holds significant national and international importance.
In Andhra Pradesh, Jawaharlal Nehru Pharma City—located in Visakhapatnam and developed by Visakha Pharmacity Ltd. (a joint venture between Ramky Group and Andhra Pradesh Industrial Infrastructure Corporation)—is India’s first pharmaceutical hub for bulk drugs. It spans 2,400 acres and hosts Pfizer, Mylan, Aurobindo, Lupin, Biocon, and many other companies 27 .
Vision Pharma: 2047
India is on the right path to capitalize on this opportune time, with key factors aligning to drive pharmaceutical manufacturing in the country. It has built a robust scientific and technological foundation through decades of extensive collaboration with pharmaceutical companies and research institutions worldwide. The government has adopted policies and initiatives, such as financial incentives, regulatory support, and infrastructure support, to drive innovation and support manufacturing and development. Plus, manufacturing costs are low and India has a strong consumer base at home.
In 2022, the Indian Department of Pharmaceuticals (DoP) introduced Vision Pharma 2047, a long-term strategy designed to transform Indian’s pharmaceutical sector into a global leader in manufacturing affordable, innovative, high-quality pharmaceutical and medical devices. They are pursuing more collaborations with pharmaceutical companies and researchers in drug development, medical devices, and digital technologies.
Although the conditions are right for growth, there are many challenges that lie ahead 28 , including regulatory hurdles, international compliance standards, protecting intellectual property, securing funding for research and development, developing and retaining a skilled workforce in the life sciences and digital technologies, building and maintaining state-of-the-art infrastructure, implementing sustainable manufacturing practices, and competing with established global companies.
The Indian government addresses these challenges, and solutions, in the Promotion of Research and Innovation in Pharma MedTech Sector (PRIP), a US$675 million scheme introduced in 2023 3 by the DoP. The ultimate goal is to shift pharmaceutical manufacturing in India from being cost-based orientated to an innovation-based model.
The ambitious vision of Vision Pharma 2047 aligns with the principle of “Vasudhaiva Kutumbakam,” a Sanskrit saying that means “the world is one family.” India aims for its pharmaceutical industry to reach a value of US$450 billion by 2047.




