ISPE and its members are developing the roadmap to introduce Industry 4.0, also referred to as the Smart Factory, to the pharmaceutical industry as Pharma 4.0™.
Mission Statement
The aim is to provide practical guidance, embedding regulatory best practices, to accelerate Pharma 4.0™ transformations. The objective is to enable organizations involved in the pharmaceutical product lifecycle to leverage the full potential of digitalization to provide faster therapeutic innovations and improved production processes for the benefit of patients.

Implementing new Industry 4.0-based manufacturing concepts in Pharma 4.0™ requires alignment of expectations, definitions, and interpretation, with global pharmaceutical regulations.
While Industry 4.0 has been called a new industrial revolution, Pharma 4.0™ implementation will more likely resemble an evolution in which digitalization and automation meet very complex product portfolios and cycles. It is therefore important to achieve an accepted understanding of readiness and maturity, starting with additional digital enablers and elements added to the ICH Q10: The Pharmaceutical Quality System along the product life cycle. It is also important to develop business cases to showcase which Industry 4.0 automation and digitalization technologies can be applied to the pharmaceutical industry and what implications are faced due to the increasingly complex regulatory challenges in the pharmaceutical and biotechnology industries.
Digitalization, an important component of Pharma 4.0™, will connect everything, creating new levels of transparency and adaptivity for a “smart” plant floor. This will enable faster decision-making, and provide in-line and on-time control over business, operations, quality, and regulatory compliance. Notably, this new connectedness will require higher levels of security, since linked systems heighten vulnerability.
Community of Practice
The ISPE Pharma 4.0™ Community of Practice (CoP) is comprised of ISPE Members engaged in implementing Pharma 4.0™ principles in their organizations or simply wanting to learn more about the concepts and network with other members interested in the topic. Membership in the Pharma 4.0™ CoP is open to all ISPE Members. For information on joining, visit the ISPE Communities of Practice page.
ISPE Pharma 4.0 Leadership Team
Steering Committee Leaders
Sub-Committee Leaders
From Industry 4.0 to Pharma 4.0™ Operating Model
ISPE’s Pharma 4.0™ CoP Steering Committee and Working Groups have developed an operating model to apply the principles of Industry 4.0 to Pharma 4.0™ which is summarized in the image below.
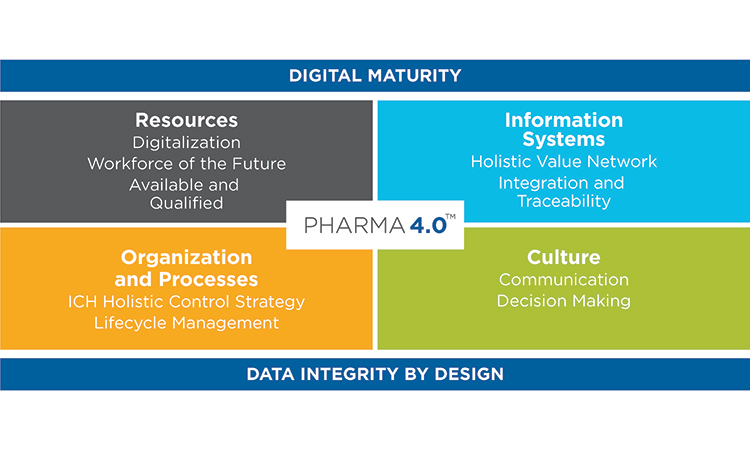
From Industry 4.0 to Pharma 4.0™ Operating Model Working Groups
- Holistic Digital Enablement
- How to provide holistic digital solutions to enable consistent, predictable, mature implementation of Pharma 4.0
- Leads: Nuha al Hafez, Roche and Yvonne Duckworth, CRB
- Process Maps and Critical Thinking
- How to develop process and data mapping to virtual models
- Leads: Volker Roeder, Arcondis and Emmie Heeren
- Plug and Produce
- Enable the transformation from centralized systems to modular, distributed, autonomous manufacturing services
- Leads: Wolfgang Winter, Agilent and Josef Trapl, Memo3 GmbH
- Memo3 GmbHValidation 4.0
- The new paradigm of a less complex validation model
- Leads: Michelle Vuolo, Tulip and David Margetts, Factory Talk
- Management Communication Strategy
- The Pharma 4.0™ elevator pitch for management
- Leads: Teresa Minero, LifeBee and Davide Smaldone, Kenvue
- Continuous Process Verification & Process Automation
- The way to parametric release
- Leads: Alicia Tébar, QBD Consulting and Miquel Romero, Almirall
Bridging the ISPE Pharma 4.0™ Operating Model with the CoP Working Groups
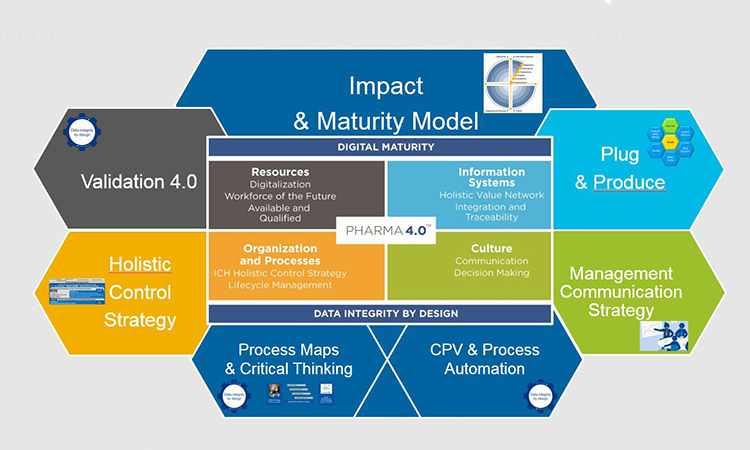
The 12 Theses for Pharma 4.0™
- Pharma 4.0™ extends/describes the Industry 4.0 Operating Model for medicinal products
- In difference to common Industry 4.0 approaches, Pharma 4.0™ embeds health regulations best practices.
- Pharma 4.0™ breaks silos in organizations by building bridges between industry, regulators and healthcare and all other stakeholders.
- For the next Generation Medicinal Products, Pharma 4.0™ is THE enabler and business case.
- For the established products, Pharma 4.0™ offers new business cases
- Investment calculations for Pharma 4.0™ require innovative approaches for business case calculations.
- Prerequisite for Pharma 4.0™ is an established PQS and controlled processes & products.
- Pharma 4.0™ is not an IT Project.
- The Pharma 4.0™ Operating Model incorporates next to IT also the organizational, cultural, processes & resources aspects.
- The Pharma 4.0™ Maturity Model allows aligning the organizations operating model for innovative and established industries, suppliers and contractors to an appropriate desired state.
- Pharma 4.0™ is not a must, but a competitive advantage. Missing Pharma 4.0™ might be a business risk.
- When moving from blockbusters to niche products and personalized medicines, Pharma 4.0™ offers new ways to look at business cases.