Decarbonizing Pharmaceutical Manufacturing Facilities

Reducing the pharmaceutical industry’s carbon footprint has become a management responsibility. This article introduces some of the key points, actual methods, and practical examples of our implementation to reduce carbon emissions from pharmaceutical manufacturing facilities in Southeast Asia.
In general drug production facilities, a hazard identification and risk assessment from a GMP perspective should always be performed on the proposed energy efficiency improvements identified from an engineering perspective, and the necessary actions should be considered. Sometimes, it is possible that a proposed improvement may not be effective or feasible. Therefore, there are considerable hurdles to reduce carbon emission from drug manufacturing plants compared to general facilities. As an example, we will describe our approach for the decarbonization at an operational pharmaceutical manufacturing facility in Southeast Asia, highlighting key steps along the way. The article also includes a note on the sustainability aspects of the sustainable development goals (SDGs).
Reducing Carbon Emissions
In recent years, reducing the carbon emissions produced by products and services has become a management task in corporate responses to climate change. Without exception, the pharmaceutical industry needs to decarbonize its manufacturing facilities and equipment. This might include a broad range of actions, including purchasing carbon credits, installing renewable energy solutions, and lowering the carbon footprint of its supply chain. In short, emissions reduction planning requires consideration of a manufacturing facility’s entire life cycle. We hope this article will provide key insights to help facilities further reduce the carbon footprint of pharmaceutical manufacturing processes.
Several approaches to low-carbon emission manufacturing facilities have been described in Pharmaceutical Engineering® articles. This article presents some key considerations, practical approaches, and examples of the reduction of greenhouse gas (GHG) emissions from pharmaceutical manufacturing facilities in Southeast Asia. The area is expected to be one of the fastest growing regions in the world regarding gross domestic product growth. The impact of GHG-related natural disasters in Southeast Asia is increasing, and international cooperation to reduce GHG emissions is expected.
Carbon Neutrality in Southeast Asia
The Association of Southeast Asian Nations (ASEAN)1 region is simultaneously exposed to many changes. It is expected to be the region most affected by future economic growth.2 increased energy consumption,3 and climate change worldwide.4
Each government in ASEAN sets a GHG reduction target (Nationally Determined Contribution [NDC]) based on the Paris Agreement framework. Table 1 describes the GHG emissions with the baseline scenario and policy scenario for ASEAN’s 10 countries.5 The baseline scenario shows the expected emissions if no emission reduction activities are implemented. The emission scenario has two cases: unconditional and conditional. The unconditional target is the country’s national policy without other countries’ actions. In contrast, the conditional target is the unconditional target subject to achieving international support relating to finance resources, technology transfer, and technical cooperation.
Furthermore, given the global trend of environmental, social, and governance (ESG) investment in the world, decarbonization actions can be evaluated and thus help secure growth resources such as capital investment.6 Based on actions consistent with these policy goals within the region and the availability of ESG investment funds, an increase in GHG-reducing actions in drug manufacturing plants in Southeast Asia is expected.
Promote Decarbonization
One way to reduce the carbon footprint of pharmaceutical products is to promote the decarbonization of manufacturing facilities. This approach to reducing GHG emissions from medical drug manufacturing facilities helps reduce the carbon footprint of pharmaceuticals and has been described in several Pharmaceutical Engineering® articles.7, 8, 9, 10 The methods for decarbonizing manufacturing facilities can be broadly divided into hard and soft measures, as described in Table 2.11
In addition, compliance with GMP is required for manufacturing conditions and operational aspects to maintain pharmaceutical quality and regulatory compliance. The location and environmental conditions of the manufacturing facility must also be considered. Therefore, the key items to understand and the required information to be gathered regarding the decarbonization of pharmaceutical manufacturing facilities are shown in Table 3. There are a wide variety of issues and considerable hurdles to achieving lowering of GHG emissions from facilities compared to less specialized general commercial and industrial facilities.
| Country | Baseline Emissions (MtCO2e) | Target Type | Target Emissions in 2030 (MtCO2e) | Gap from Policy Scenario (MtCO2e) |
|---|---|---|---|---|
| Brunei Darussalam | 13.9 | UC | 10.3 | 4 |
| C | 10.3 | 4 | ||
| Cambodia | 15.3 | UC | 15.3 | - |
| C | 11.2 | 4 | ||
| Indonesia | 1,450.3 | UC | 1,218.2 | 232 |
| C | 1,160.2 | 290 | ||
| Lao PDR | 22.5 | UC | 22.5 | - |
| C | 21.4 | 1 | ||
| Malaysia | 544.4 | UC | 539.8 | 5 |
| C | 456.8 | 88 | ||
| Myanmar | 72.8 | UC | 72.8 | - |
| C | 69.6 | 3 | ||
| Philippines | 293.1 | UC | 293.1 | - |
| C | 87.9 | 205 | ||
| Singapore | 51.4 | UC | 65.0 | -14 |
| C | 65.0 | -14 | ||
| Thailand | 645.0 | UC | 516.0 | 129 |
| C | 483.7 | 161 | ||
| Vietnam | 570.9 | UC | 525.3 | 46 |
| C | 428.2 | 143 | ||
| ASEAN | 3.679.6 | UC | 3264.7 | 415 |
| C | 2780.7 | 899 |
UC: unconditional, C: conditional
Note: Estimates exclude LULUCF-related emissions. Country emissions may not sum to the
ASEAN totals due to rounding.
| Category | Methods | |
|---|---|---|
| H1 | Hard measure | Shifting to the low-carbon energy source (solar, hydro, wind, geothermal, nuclear, etc.) |
| H2 | Hard measure | Installing new or renovating existing equipment to improve energy e ciency |
| S1 | Soft measure | Bridging the gap between required and actual operating conditions in response to increased or decreased production, and/or assessing the utility requirements out of the utility capacity to optimize system operation |
| S2 | Soft measure | Setting lower operating conditions during nonworking hours, including nighttime or nonoperation hours for production lines |
| Category | Essential Subjects and Information | |
|---|---|---|
| 1 | Regulations related to GMP | • GMP in the region and regions the facility supplies • Pharmaceutical quality system and validation practices at the subject facility |
| 2 | Regulations related to decarbonization and environmental protection | The latest regulations related to GHG reduction and environmental protection in the area where the subject facility is located |
| 3 | Building design | Optimal layout design and thermal performance |
| 4 | Heating, ventilation, and air conditioning (HVAC); electricity; and utility design | Optimal system design and operational control |
| 5 | Production equipment/ processes | • Pharmaceutical quality management and manufacturing conditions • Production procedures and operating methods during manufacturing at the facility |
| 6 | Decarbonization and energy-saving technologies | The latest technologies and trends in GHG-reducing products and services |
| 7 | Pharmaceutical manufacturing technologies | The latest technologies and trends in production/ utility facilities |
| 8 | Other environmental conditions | Environmental conditions (including costs) in the area of the facility |
Source12
Approaches and Decarbonization Examples
In this section, we consider the entire manufacturing facility and present a systematic approach to decarbonize pharmaceutical manufacturing plants in Southeast Asia. Pharmaceutical manufacturing facilities, being business organizations, should follow a management system. The main stages are presented next, based on ISO 50001:12 Management commitment, energy policy, developing execution plan, plan execution, and evaluation of performance (see Figure 1).
Management Commitment and Energy Policy
A facility’s management organizes the person and/or team responsible for carrying out energy management activities and establishes an energy policy that articulates its commitment to achieving improvements in energy performance. Based on the energy policy, energy management activities are planned and implemented. The management also provides financial and human resources to realize its commitments.
Develop a Plan
An energy audit should be conducted to determine the entire manufacturing facility’s energy supply and consumption status. This process will include proposals for specific measures to improve energy efficiency and reduce costs based on energy usage status. It consists of the following steps.
Step 1: Collect facility data
Collect ongoing facility and energy-related data from the facility’s engineering department. Multiple years of data are preferred to account for seasonality and longer-term trends. It may be necessary to take supplementary measurements or even add instrumentation to assess specific leads.
The data items should include product items and their specifics, manufacturing process information, an overview of the facility and equipment, cost data for energy sources to be procured, types of energy consumed, and energy consumption data for each facility (production machinery, utilities, HVAC equipment, etc.).
Step 2: Review preliminary considerations
Based on the data collected, a desk-based energy consumption analysis should be conducted to examine options from a comprehensive perspective of efficient use and waste reduction. Using Sankey diagrams can be very helpful when sharing or reporting energy-related information. Examples of basic options follow.
- Change to a more energy-efficient utility system if the plant is equipped with its own power generation or steam generator
- Change the energy source to a lower- or zero-carbon option
- Retrofit or upgrade to higher-efficiency equipment or equipment components
- Minimize water consumption by reducing use or reuse
- Review operational states and consider intermittent use (turning off), silent hour setback, and campaign working
Based on the preliminary study results from step 2, conduct field inspections. The following are examples of items to be checked in the field: verification of preliminary considered options; age of the facility, maintenance records, and history of facility modifications and changes; up-to-date facility, systems engineering drawings, and documentation; verification of the environmental conditions of the manufacturing environment, especially the performance of the HVAC system; and identification of waste and inefficient areas and operations of the facility from an energy consumption perspective.
Step 3: Identify hazards to GMP and address risks
Two key activities are defined by ISO 50001:12 the plan-do-check-act (PDCA) cycle to achieve efficiency in energy use, and the PDCA cycle to ensure compliance with energy-related regulatory requirements. In pharmaceutical production facilities, the environmental conditions under which products are manufactured and maintained in pharmaceutical production facilities are strictly regulated by law and GMP, and compliance is mandatory.
For every energy efficiency improvement idea identified from an engineering perspective, a hazard identification and risk assessment must be performed. This should be done from a GMP perspective, and the necessary actions must be considered. It is not unusual to conclude that the improvement proposal may not be feasible, effective, or practical, or that it presents too great a risk. In addition, a change of control must be prepared for all planned changes. The necessity and scope of requalification and revalidation must be discussed and approved by a quality assurance (QA) manager, and appropriate qualification and/or validation must be performed.
A characteristic of drug production plants is that they are often overdesigned initially to allow for factors such as performance safety margin, reserve capacity for changes in use or occupancy, and expansion. Additionally, production changes, increases, and decreases from the originally anticipated manufacturing plan lead to a high probability of a significant discrepancy between the design intent and the current facility utilization. By understanding the divergence between these, it is possible to identify opportunities to remove excess equipment or adjust and regulate systems to reduce excess capacity specifications. There are two different perspectives for reviewing these matters: a review of production environmental parameters and a review of operational management.
Review of production environment parameters
Review the latest production environmental control performance parameters, including clean room ventilation air changes, air supply/exhaust volume, room temperature, relative humidity, and room pressure differential settings. Adjustments should be made to the extent that the changes do not affect quality, thereby improving productivity and reducing energy consumption and carbon emissions.
Review of operational management
Encourage energy conservation by reviewing facility operation and management during nonoperating periods. Significant energy savings can be achieved by shutting down systems during nonproduction periods, setting back performance during production idling mode, closing steam lines when they are not required due to seasonal needs, etc.
Step 4: Prepare an energy audit report
The energy audit report is prepared by analyzing the results to date. Its structure is as follows. First, energy consumption is analyzed, including for the entire manufacturing facility, all processes and staff, and each system within the facility. Second, comprehensive engineering proposals are created to improve the efficiency of the energy used. Third, inefficient energy use is identified and ways to address it are developed. Fourth, the magnitude of capital investment required and the resulting energy conservation reduction effects are considered. If appropriate, the investment payback period and return on investment (ROI) should be defined and considered. It should be noted that carbon reduction measures, such as changing the energy source from gas or oil to electricity, may not save money and may cost more to operate.
For every energy efficiency improvement idea identified from an engineering perspective, a hazard identification and risk assessment must be performed. This should be done from a GMP perspective, and the necessary actions must be considered.
Typical project categories include proposals with:
- Small or no additional investment and an energy reduction target of about 5%, e.g., equipment optimization tuning
- Moderate investment and an energy reduction target of 5%–15%, e.g., introduction of new technology and high-efficiency equipment
- Large investment and an energy reduction target of 15%–30%, e.g., introduction of new technology and high-efficiency equipment renewal of aging facilities
An energy audit usually takes about two months from the start to the submission of the report. Analysis and consideration of options are difficult when insufficient materials for analysis are available or when energy use and consumption are not visible. An estimation based on decarbonization and energy-saving technologies given in Table 3 may also be useful in this situation.
The report should provide a status report and a future vision of carbon neutrality in manufacturing facilities for the management of the company owning the facility and the facility’s management. The process of preparing the energy audit and report, which is a collaborative effort with the employees of the manufacturing facility, can be seen as an introduction to a series of hypotheses, considerations, and implementation of solutions for continued energy efficiency and carbon reduction over time.
Step 5: Review and rank implementation recommendations
The faculty management personnel should review the current situation at the target facility and proposals for improving energy efficiency internally to determine whether they should be implemented. Management will select the proposals for implementation based on each proposal’s ROI or carbon savings in a manner linked to the production, capital investment, and maintenance plans.
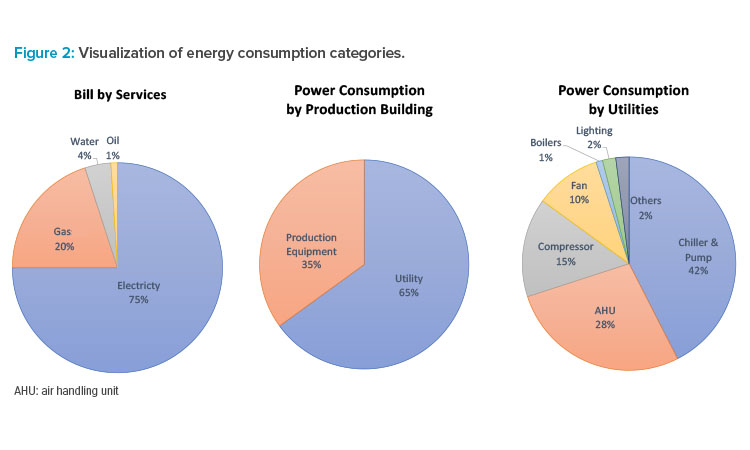
In addition, as indicated in the report, the effort to go carbon neutral for a manufacturing facility will be long term, so the capability of energy consumption visualization will be essential (see Figure 2). The factory energy management system is a useful numerical dashboard for considering and implementing carbon neutrality measures, as both a building management system and an environment monitoring system become essential systems in the facilities.
Step 6: Prepare for and implement changes
After the management team reviews and ranks the proposals, they should be organized and implemented as a project. Capital investment should then be made based on the payback period, if applicable. This includes life cycle costs, energy-saving measures, and production efficiency improvements. In doing so, financing methods such as leases, rentals, and subsidies should be considered.
Plan Execution, Monitoring, and Follow-Up
The project is executed in accordance with the PDCA cycle, which includes the process evaluation based on the measured results. This article focuses on the planning process, which is the most essential aspect to consider in terms of numbers. Efforts to become carbon neutral in the plants require a long time. Therefore, achieving incremental CO2 emissions reductions will require continuous reductions in inefficient energy consumption in equipment and operations throughout the facility. The series of tasks outlined previously should be repeated to achieve this reduction. In addition, the information on the decarbonization of pharmaceutical manufacturing facilities listed in Table 3 requires continuous follow-up activities because they change with technological advancements, regulations, etc.
Decarbonization and GMP Compliance
We will now consider the decarbonization of two specific systems. These are HVAC and pharmaceutical water systems that are critical from a GMP perspective. Both systems have significant energy and GHG impact.
HVAC System
While the HVAC system in pharmaceutical plants is an important system for maintaining a high level of manufacturing environment control, the energy savings associated with changes in equipment and operating conditions can be significant. Therefore, conducting a risk assessment for each feasible energy-saving item is important to see if GMP-like problems arise. For example, will the differential pressure between rooms be maintained if the ventilation rate is reduced? Can the cleanliness level be maintained? Will it affect temperature and humidity control? During the evaluation and assessment, it is important to review the HVAC and system control diagrams with the engineer, identify risks jointly, and determine avoidance measures and recommissioning or requalification requirements.
First, it is important to establish the required air volume supply requirements. That may be expressed as numerical air changes–rate per hour based on airborne cleanliness level and contamination source strength load, heat gains in the occupied space, and recovery times specified in GMP guidance documents such as Annex 1 of the PIC/S GMP. ISO 14644-1613 highlights that the airflow contributes significantly to the cleanroom’s energy consumption. Therefore, reducing airflow rate leads to significant energy savings. Theoretically, the ventilation power by fans depends on the cube of the airflow rate,7 so if the airflow is reduced by half, electrical consumption by fans is reduced by one-eighth (see S1, S2 from Table 2). Therefore, introducing nighttime and holiday modes, which reduce the air flow rate during nonoperating hours such as nighttime and holidays, has a significant energy-saving effect.
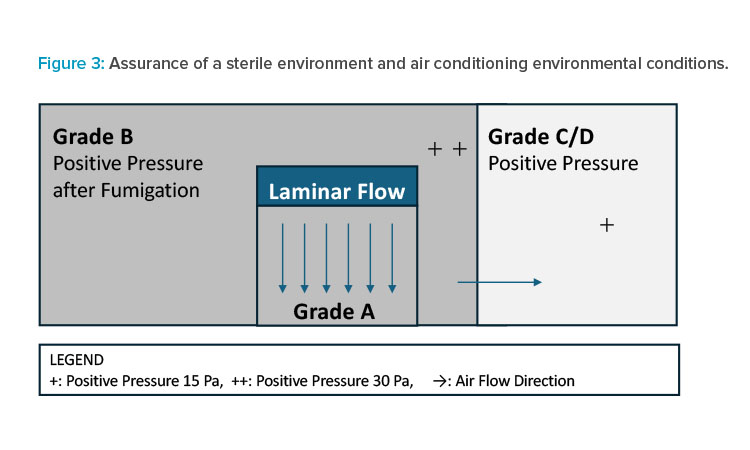
When considering aseptic drug product manufacturing facilities, the reality is that sterility assurance is the most critical issue, and reducing the risk of loss of aseptic processing conditions is achieved by ensuring robust conditions. To save energy, it is advisable first to consider whether it is feasible to shut down the air conditioning during nonoperating periods in Grade C/D, which occupy the least amount of cleanliness in the largest area (see S2 in Table 2). Consideration could be given to whether it is possible to reduce the air change rate during nonworking hours in Grade B while maintaining room pressure. It may also be necessary to consider whether it is feasible to shut down unidirectional Grade A airflow during nonoperating periods to achieve further energy savings (see S2 in Table 2).
In many cases in Southeast Asia, aseptic operations are still conducted under conventional Grade A unidirectional airflow, and aseptic assurance in conventional Grade A and Grade B facilities usually employs periodic bio-decontamination based on sterilization. This means stopping the airflow is unlikely to be acceptable due to the significant time required to re-establish aseptic conditions. A more practical approach would be reducing velocity during silent hours, ensuring that the Grade A cleanliness is maintained in a rest state.
Many QAs consider a sterile break as soon as the laminar flow is stopped during nonoperating hours. This is because it is almost impossible to validate the reproduction of an aseptic environment when the laminar flow is restarted by any method other than sterilization (see Figure 3). In this case, it is a balance between the energy-saving effect, the labor required for verification, and the risk of aseptic assurance. Thus, although the operation of air conditioning equipment during nonoperation is a huge energy-saving target item, some issues need to be resolved to apply soft measures to a sterile environment.
Pharmaceutical Water Utilities
Water used for the manufacture of sterile products, water for injection (WFI), is required to be highly controlled for microbial levels, endotoxins, total organic carbon, and conductivity. The water, frequently produced by high-temperature distillation, is maintained over 80°C/176°F14 within the storage and distribution system to inhibit microbial growth and then cooled for use during processing. Therefore, energy consumption for heating and cooling is significant, and alternative approaches should be considered to save energy and associated CO2.
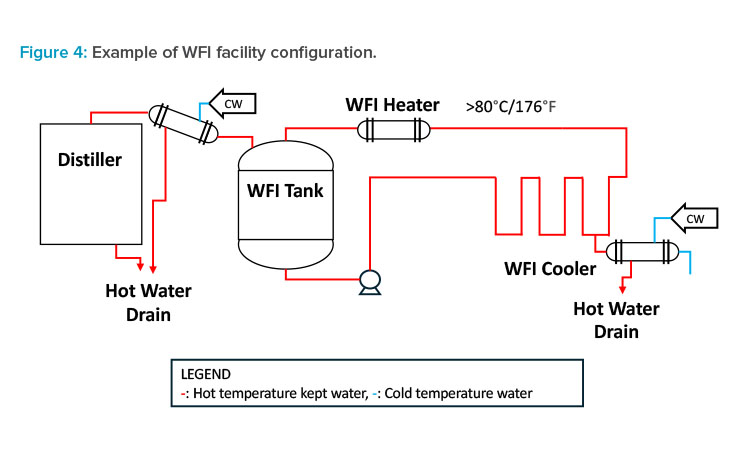
Pharmaceutical water systems consume energy to maintain pharmaceutical water quality. Therefore, the energy conservation policy is to build a system that uses as little heat source as possible, and to reduce the amount of WFI used as much as possible and reduce the capacity of the system.
Now that the major pharmacopeias (US, Europe, and Japan) have been revised to accept the technology of room temperature membrane-based systems, there is an opportunity to adopt this system. We have the opportunity to change to ambient temperature membrane-based systems because most pharmacopeias have been revised to accept this technology. These systems operate in conjunction with the ozonation of the storage and distribution system to inhibit microbial growth. Such systems obviate the need to provide steam heating and high-capacity cooling utilities.
When WFI is produced by distillation, heat source steam is used, and a large amount of cooling water is used in the distiller’s condenser. The produced WFI is kept hot (> 80°C/176°F) in the WFI tank and circulation loop and cooled by a local cooler at the time of use (see Figure 4). The wastewater from each process is mixed with high and low temperatures and discarded as medium-temperature wastewater, making heat recovery difficult. However, this can be designed in advance to recover heat from wastewater, although additional equipment for heat recovery and the cost-effectiveness of heat recovery must be considered.
When distillation-based WFI systems are employed, it is possible to design a system that uses heat recovery in a manner that does not conflict with GMP. However, additional equipment for heat recovery and the cost-effectiveness of heat recovery should be considered. Reducing the consumption of WFI is also a mechanism for energy reduction. Experience tells us that the amount of WFI used for cleaning is often greater than the aqueous component of the product.
In this case, the following examples can be considered.
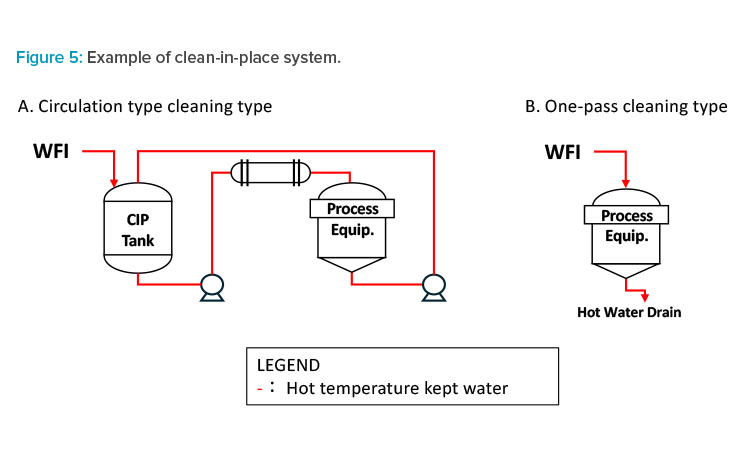
- Reducing washing water by switching from one-pass washing (see Figure 5B) to circulating washing is possible but involves large-scale production facility changes (see Figure 5A).
- It is possible to reduce cleaning water by reducing unnecessary cleaning time with regard to the construction of changes to production facilities. Reducing wastage due to excessive cleaning time from the safety side approach during validation is proposed as one of the methods.
Therefore, reducing usage by a reduction in cleaning time entails reconfirming cleaning effectiveness (cleaning validation).
Becoming a Sustainable Facility
We recommend, and emphasize, three perspectives when realizing the approaches we have discussed for promoting the GHG reduction from drug manufacturing facilities and examining risks from a GMP perspective.
Long-Term Commitment
Achieving decarbonization of a drug production facility requires optimization of CO2 emissions, energy consumption, and product output throughout the facility’s life cycle period. The facility’s life cycle cost includes energy consumption but also maintenance, renewal of facilities, and more. Therefore, it is essential to understand the effects and costs during the long-term life cycle period.
The Human Factor
Because this is a companywide initiative and must be based on a long-term plan, the top management leadership of the manufacturing facility is paramount. Disclosure of the initiative to all staff, progress based on consensus, and reflection as a management indicator are desirable. The hurdles to optimizing operations and higher quality from the perspective of quality first and GMP validation are high.
Communication between quality control, production, and facility management departments is essential, and management commitment is critical. The optimization of energy usage will be encouraged based on the optimization of the production system. Proposals for the renewal of aging facilities by the facility management department will be more effective if the payback on investment based on optimized energy usage is considered.
Master Plans Through the Life Cycle
Master planning through the life cycle period and forecasting can be used to promote ongoing optimization. The master plan includes annual production targets, CO2 reduction measures and targets, energy consumption targets, payback (if applicable), replacement plans for aging equipment, and maintenance (including overhauls). Optimization needs to be reviewed each time because production status and items change. The master plan is vital as a basic guideline in such cases.
However, to pass on manufacturing facilities to the next generation, we must not forget the sustainability perspective of the SDGs as we work to reduce GHG emissions. Manufacturing facilities undergo numerous upgrades over the decades, and their carbon footprint must be reduced over their life cycle.
If the plant is in a country that is on track to achieve carbon neutrality by 2050, synchronize the plant’s long-term roadmap to decarbonization with the policy goal.
Adapting Local Circumstances
If the plant is in a country that is on track to achieve carbon neutrality by 2050, synchronize the plant’s long-term roadmap to decarbonization with the policy goal. Product items and other factors manufactured in factories tend to change more frequently in response to recent technological innovations, economic growth, and geopolitical balances. Therefore, long-term efforts will need to be reviewed throughout the life cycle, and the methods that are appropriate to the production situation should be continually sought.
Southeast Asian plants have an annual cooling load with no winter heating due to high year-round temperatures. Based on these related temperature characteristics, the key points are to reduce the amount of outdoor air treated (with high enthalpy throughout the year) and to reduce the cooling load. In the future, climate change will likely require consideration of load increase in response to temperature and pressure changes in extreme weather.
Conclusion
In addition to achieving carbon neutrality, reducing the carbon footprint of pharmaceuticals is a management issue of increasing importance. This article presents some of the considerations, practical methods, and examples of practices we have implemented to decarbonize pharmaceutical manufacturing facilities in Southeast Asia, an area expected to be one of the fastest growing regions in the world regarding gross domestic product growth.
In similar facilities, it is always necessary to conduct risk analysis from a GMP perspective and consider necessary measures for the energy efficiency improvement proposals identified from an engineering perspective. Depending on the circumstances, it is possible that declining a proposed improvement may not be effective or feasible. Therefore, there are considerable hurdles in achieving GHG reduction from drug production plants compared to general facilities.
Our team presented an approach and examples study of decreasing GHG emissions at an operating pharmaceutical manufacturing plant in Southeast Asia. The main steps involved assessing the current situation, listing and prioritizing measures, installing equipment, changing operating conditions and validation, and following up with ongoing monitoring.
In promoting decarbonization, the sustainability perspective of the SDGs for carbon footprint reduction from a manufacturing facility must also be considered. The carbon footprint reduction also requires a long-term incremental reduction plan considering the manufacturing facility’s life cycle. Implementing a carbon reduction plan will be a long and arduous journey, but we would like to offer encouragement for those working to implement similar measures in their facilities: It can be done!

![Figure 1: Approaches to realization and examples of decarbonization [12].](/sites/default/files/pe/2024/Nov-Dec/1124_PE_ND_Goto_02.jpg)