A Proposal for a Comprehensive Quality Overall Summary

When working with the common technical dossier (CTD), the structure of Module 2 “follows the scope and outline of the Body of Data in Module 3,”
This novel approach to Module 2 uses a structure that shows how enhanced process knowledge, product understanding, and risk assessments are linked to the control strategy. Application of this innovative approach will quickly orient regulators to the content of Module 3, “present product quality benefit-risk considerations, summarize the pharmaceutical development, present an overall understanding of the product quality,” 1 and facilitate continuous improvement.
As part of the US Food and Drug Administration (FDA) QbD pilot4 in 2005–2006, the need to convey how the control strategy is linked to the target product profile (TPP) and quality target product profile (QTPP) was discussed. One possible solution raised was a Module 2 that integrates the product development story, links the product attributes to the drug product (DP) and drug substance (DS) manufacturing processes to demonstrate a holistic drug product control strategy, and demonstrates how the control strategy supports the TPP.
Ultimately, the summary was not adopted during the QbD pilot, but was reintroduced in 2014 when a Pharmaceutical Research and Manufacturers of America (PhRMA) team prepared a white paper describing the content and format of an improved Module 2. A draft of the paper was shared with several regulators, and it was agreed that there would be value in an overview of the product to familiarize reviewers with the comprehensive development story and control strategy prior to examining a particular element of the CTD. The proposal for the Quality Overall Summary (QOS) resembled what constituted the Expert Report5 in Europe prior to the adoption of the CTD.
This proposal is also somewhat aligned with the concise, logical framework of the Japanese Gaiyo, but is further intended to provide assessors with a well-articulated introduction to key aspects of the overall control strategy and narrative of how the data contained in Module 3 and associated risk management justifications are reflected in the regulatory binding elements described in the Japanese Application Form.6
Based on the framework that was developed by the PhRMA team, Pfizer piloted an approach to Module 2 that was referred to as the “comprehensive Quality Overall Summary (QOS).” While a comprehensive QOS is not currently defined by International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), it is a Module 2 summary document that contains a QTPP, a TPP, a development narrative and resulting control strategy, and a listing of regulatory commitments/ECs.
The objectives of the comprehensive QOS format are to describe the product development process in the context of assuring quality and mitigating risk to the patient, to provide a clear summary of the control strategy, and to guide the reviewer through the content of Module 3. The comprehensive QOS was successfully submitted to all global markets with the exception of Japan where the Module 2 format is very specific. Direct feedback from the US FDA on this pilot is shared in the discussion.
This article summarizes an alternate and more functional way to format the QOS presented in Module 2.3. In addition, the ISPE Regulatory Quality Harmonization Committee (RQHC) regional focus group is developing proposals on the content and structure of a risk-based Module 2 that could ultimately support standardization of chemistry, manufacturing, and controls (CMC) terminologies and submission standards for control strategy harmonization and cloud assessment.7,8 Both of these efforts could serve to provide options to the Expert Working Group (EWG) that is currently developing strategies for the revision of The Common Technical Document for the Registration of Pharmaceuticals for Human Use: Quality–M4Q(R1), Quality Overall Summary of Module 2, Module 3: Quality.9
Among other things, the M4Q(R2) EWG is working toward “organizing product and manufacturing information in a suitable format for easy access, analysis, and knowledge management.”1 The ICH M4Q(R2) concept paper sets goals for “better capturing the pharmaceutical development and the proposed control strategy, which should be the backbone of the revised M4Q structure. This should address key elements of the proposed pharmaceutical product, including the QTPP, manufacturing process, and control strategy.”1
Considering the ongoing work within ICH to revise the quality section of Module 2.3 and the implementation of ICH Harmonised Tripartite Guideline Q12: Technical and Regulatory Considerations for Pharmaceutical Product Lifecycle Management, which brings focus on the control strategy when reviewing post-approval changes, now is a good time to discuss the format of Module 2. A comprehensive QOS that clearly articulates the product link to the patient and presents and justifies the product control strategy from beginning to end would be valuable.
Challenges with the Current Module 2 Structure
The US FDA published a white paper in 2018 calling for a revision to Module 2 because, “there can be a disconnect between applicants and regulators regarding the communication of quality data and its impact on the assessment. Currently, it takes time and/or communications (e.g., information requests) to fully understand the quality data and its significance in an application.”10 The FDA suggested that it would be valuable to connect the summary to the patient, describe the control strategy, and guide the regulator through the submission. Further, they indicated that there is an opportunity to present and discuss life cycle management plans for the product. Addressing these points would allow the regulator to “be prepared to best assess the applicant’s own conclusions about potential risk to the patient, and the control of such, in the commercially manufactured product.”10
The current use of Module 2 to summarize Module 3 creates challenges for both regulators and industry. The regulators are not provided with a coherent and complete description of a product control strategy or overview of the life cycle change management. Companies who are market authorization holders (MAHs) must manage inconsistency in regulatory assessments among global regulators,11 and must provide additional information due to the lack of integration between inspections and assessments, and have no incentive to use enhanced development approaches for post-approval changes. Module 2 could serve as a location in the CTD structure to effectively describe the control strategy, which could lessen significantly the effort required to understand how the control strategy fits together and lay the foundation for MAHs to seek flexible regulatory approaches that support an efficient life cycle management plan.10
Module 2 for a new drug registration application typically consists of individual summaries presented in the same order as those in the corresponding Module 3 section [8]. Because the story that underwrites the control strategy is not presented until the second major subsection of Module 3 (i.e., Section 3.2.S.2.6 or 3.2.P.2 for DS or DP, respectively), reading the dossier and/or summary in the sequential order of the CTD can reduce review efficiency, particularly when a QbD approach is employed. Furthermore, elements of the control strategy are spread across several sections of the CTD and are not easily linked to the development narrative and comprehensive risk assessments. This current Module 2 may not provide a holistic summary nor an insightful view into the control strategy or product development.
At present, there is a concerning trend away from global harmonization of Module 2. National regulatory agencies are requesting bespoke summary documents, such as the Canadian Certified Product Information Document, the Japanese Application Form, the Korean CMC Summary Document, and the South African Summary of Critical Regulatory Elements, as discussed in the article by Kendsersky et al [8]. In that article, the authors outlined an opportunity to propose a summary document that “frames the drug development story by effectively conveying how enhanced process understanding, product knowledge, and risk assessments are linked to a comprehensive control strategy; links the drug substance and drug product critical quality attributes (CQAs) to target product profile (TPP) and quality target product profile (QTPP); summarizes the holistic control strategy, including links to more detail in Module 3, demonstrating how the proposed manufacturing process and controls (namely, critical process parameters, critical material attributes, and ECs) will provide assurance a drug substance and drug product will meet their respective CQAs; and declares and documents a summary of the ECs, and a proposed Product Lifecycle Management (PLCM) document,3 if applicable can also be leveraged.” [8]
The proposed comprehensive QOS fulfills these conditions. If elements such as these are adopted during the revision of ICH M4Q, this could influence global regulatory agencies to eliminate the requirement for custom Module 2 commitment documents.
Proposed Solution
The control strategy for each individual product will be unique, as it is based on the properties of the individual DS, formulation, and manufacturing processes and, if applicable, devices. The comprehensive QOS provides a single location to bring together the development narrative, the control strategy, the ECs, and a suggested life cycle approach to change them. The recommendations outlined within reflect the successful elements of the 2018 comprehensive QOS pilot strategy with the added objective of further streamlining the summary into a more concise and ordered format.
Figure 1 illustrates the interconnectivity of how the product is designed and quality is controlled to deliver the needs of the patient. A well-written, globally harmonized comprehensive QOS that includes a development narrative would provide regulators with a concise, science- and risk-based development story that highlights a product’s control and life cycle strategies, thereby potentially decreasing review and approval timelines and enabling faster availability and sustained supply of critical medicines to patients worldwide.
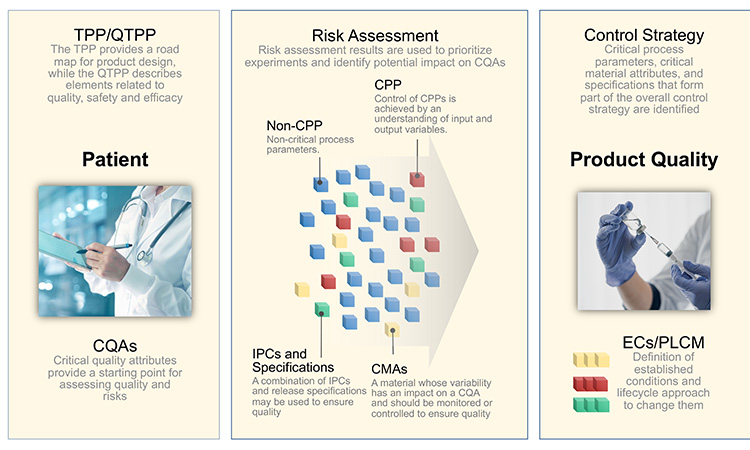
TPP/QTPP and Definition of CQAs
As illustrated above, a development narrative briefly summarizes the quality elements of the product that are designed to meet patient needs (TPP and QTPP) and is organized based on the product understanding, risk assessments, and development data used to justify the control strategy. The information contained in the narrative will provide an integrated summary that shows how and why important aspects of the control strategy that are detailed in the ECs were selected.
Starting with an understanding of the needs of the patient and the TPP provides the reviewer a roadmap of the product design and development, while providing a clear linkage to the patient. The TPP defines the indication and patient population, describes usage of the product, provides dosage and administration details, explains dosage form and strengths, and lists packaging and storage requirements.
A preliminary list of the CQAs derived from the TPP provides a starting point for assessing the risks associated with product quality. The purpose of the QTPP is to link the attributes of the DP back through the TPP to the needs of the intended patient population. The QTPP describes elements of the product related to quality, safety, and efficacy, as shown in Table 1.
| Patient Considerations | Product Design Considerations | Target Attribute | CQA | Label |
| Treatment of patients with breast cancer with ability to titrate dose | Three strengths of tablets differentiated by size and debossing | 5, 10, and 15 mg film coated tablets | Appearance | CQA1 |
| Identity | CQA2 | |||
| Confidence in quality of medicine | Consistent quality with each dose | Meet pharmacopoeia requirements for potency and content uniformity | Assay | CQA3 |
| Content Uniformity | CQA4 | |||
| Oral once per day dosing | Immediate release oral dosage form | Rapid in-vitro drug release | Dissolution Rate | CQA5 |
| Easy to open & adequate shelf life | Easy to open with minimum 36 months at 30oC/75%RH | Packaging that protects product over intended shelf life, including HDPE bottle with desiccant | Degradation Products | CQA6 |
| Water Content | CQA7 |
The patient considerations drive the product design considerations and target attributes. From there, a preliminary list of CQAs is identified. For purpose of illustration used throughout this article, labels have been assigned to each CQA. This list of CQAs provides a starting point for assessing the risks associated with product quality.
Pharmaceutical Development
The important aspects of the pharmaceutical development illustrated in Figure 2 will be summarized in the narrative of the comprehensive QOS. According to ICH Harmonised Tripartite Guideline Q8 (R2): Pharmaceutical Development, “Potential drug product critical CQAs derived from the quality target product profile and/or prior knowledge are used to guide the product and process development. The list of potential CQAs can be modified when the formulation and manufacturing process are selected and as product knowledge and process understanding increase.”12
The CQAs are used as a basis for an initial risk assessment to determine areas that may warrant investigation through an experimental plan to determine safety and efficacy risks to patients. The initial risk assessment is based on information on the target compound as well as prior knowledge and experience from other products with similar characteristics. The results of the risk assessment are used to define experiments.
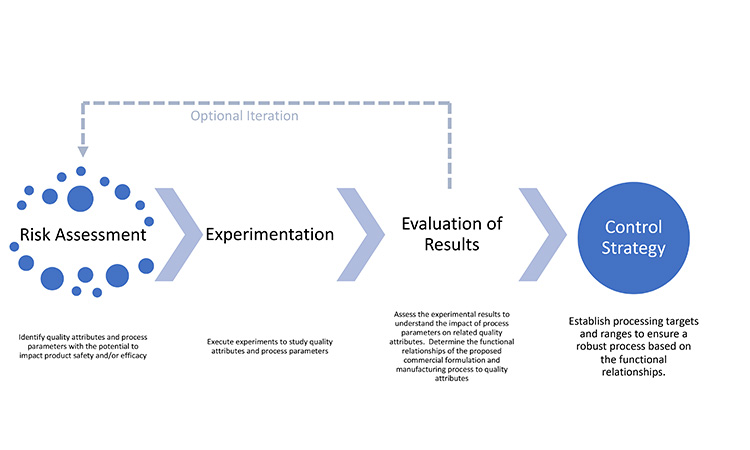
This assessment is performed for each CQA in turn, identifying parameters or variables that have the potential to affect those attributes. The results of the risk assessment are used to define and prioritize experiments. Results from experiments may confirm or modify preliminary risk assessments. Table 2 shows an example of a finalized risk assessment after experimentation, where items highlighted in yellow and red are described in the regulatory binding elements of the control strategy. Standardizing risk assessment summaries reported in applications will increase transparency and facilitate agreement between global regulatory authorities and industry on appropriate and acceptable control strategies.
Table 2: Example of a final risk assessment summary table.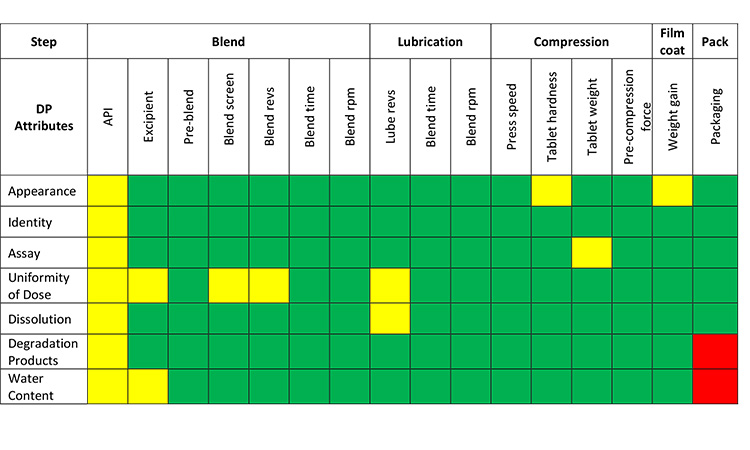
Key:
- Green: Parameters or material attributes have no relationship to a CQA. Non-critical controls are in place.
- Yellow: Parameters or material attributes have a relationship to a CQA. Critical controls (ECs) are in place.
- Red: Parameters or material attributes have a relationship to a CQA and an edge of failure has been identified. Critical controls (ECs) are in place.
The iterative cycle of risk assessment and experimentation can be considered complete when the relationships between parameters and CQAs are understood and take into consideration the broader context of the clinical significance of CQAs and the ability to control them during processing.13 Transparent communication of the relationship between CQAs, risks, and mitigation strategies will benefit the regulator assessing the application as well as the MAH assessing potential post-approval changes.
A summary of pharmaceutical development that presents the knowledge gained through the application of scientific approaches and quality risk management during the development of a product and its manufacturing process12 provides substantiation for the regulatory binding information that define a product control strategy. Provisions for additional detail may be established by hyperlinks to Module 3 within the CTD.
Presenting the Control Strategy
A control strategy consists of “a planned set of controls, derived from current product and process understanding that ensures process performance and product quality. The controls can include parameters and attributes related to drug substance and drug product materials and components, facility and equipment operating conditions, in-process controls, finished product specifications, and the associated methods and frequency of monitoring and control.”12 Elements of a control strategy may be parametrically based or may be primarily focused on control of process outputs (e.g., attributes, measurements, responses).3 Control of a CPP and/or CMA is achieved by understanding the relationship between input and output variables in a manufacturing process, including material attributes, in-process controls and process conditions, and operating parameters. Every CPP (CMA and CQA) represents regulatory binding information.
While CMA has not been endorsed as an ICH term, it is being widely used to distinguish the variability associated with conditions in a process, known as process parameters vs. variability inherent in materials used in the process, known as material attributes. Regulatory binding information may also include non-CPPs and non-CMAs in addition to CQAs and CPPs. The distinction between critical and non-critical attributes and parameters will be necessary to ensure regulatory oversight of post-approval management is appropriately differentiated.
Specific controls can be established within the manufacturing process where the boundaries are defined by the inter-relationship between process parameters. Specification criteria are often established to control CMAs. Analytical methods are developed and adopted to evaluate specific materials—i.e., raw materials, intermediates, DS, formulation, and packaging components, DP—against pre-defined specification criteria to confirm control. While all controls are intended to assure quality product, finished product testing, by itself, does not constitute a control strategy.
The comprehensive control strategy can be visualized in the pictorial diagram shown in Figure 3. The backbone of the control strategy is made up of the CQAs shown as red boxes. Process parameters, material attributes, in process tests, release tests and GMP controls which are critical are represented by red ovals, while those that are non-critical are represented by blue ovals. The diagram shows that some elements of the control strategy have primary functional relationship to the CQAs, while others may have secondary, tertiary, or beyond. Further, it is shown that some CPPs may impact more than 1 CQA. For example, CPP15, which is the number of rotations used during lubrication blending, impacts CQA4 (content uniformity) and CQA5(dissolution). Other such examples are apparent in the diagram. It is envisioned that critical elements of the overall control strategy mapped in Panel B would be described in Module 2.
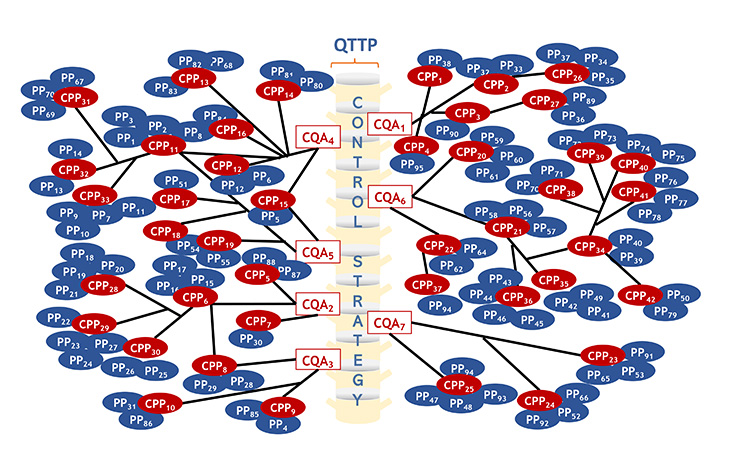
Panel A: Functional relationships among CQAs and various process parameters, material attributes, IPCs, tests and GMP Controls. The details underpinning this would be described in Module 3.
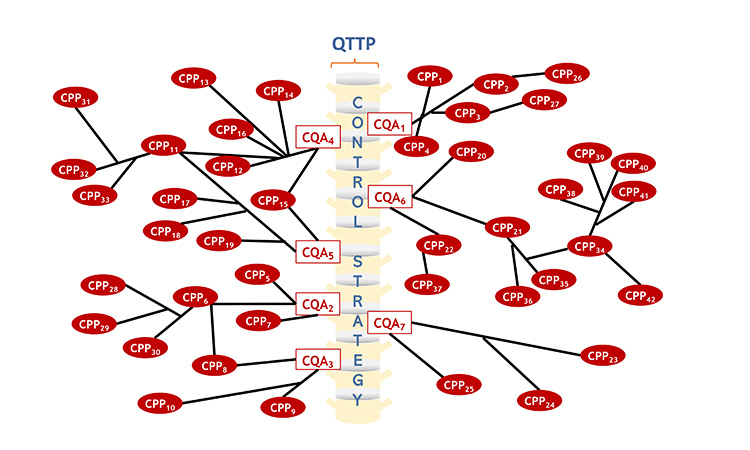
Panel B: Functional relationships among critical elements of the control strategy. The elements of the overall control strategy shown below would be described in Module 2.
This control strategy diagram illustrated in Figure 3 can be translated into a tabular summary of the product control strategy showing the functional relationship between DP CQAs and CPPs (Table 3).
| Product Attribute | CQA | Functional Relationship | Controls | ||
| Route of Administration/ Dosage Form | Appearance CQA1 |
CQA1 = f (CPP1 CPP2 CPP3 CPP4) CPP2 = f (CPP26) CPP3 = f (CPP27) |
CPP1 | CMA | DS appearance (S.4.1) |
| CPP26 | GMP | Compression tooling (PQS) | |||
| CPP2 | IPC | Tablet hardness (P.3.4) | |||
| CPP3 | IPC | Film coat weight gain (P.3.4) | |||
| CPP27 | GMP | Film coat dispensing (PQS) | |||
| CPP4 | Test | Appearance (P.5.1) | |||
| Potency Identity | Identity CQA2 |
CQA2 = f (CPP5 CPP6 CPP7) CPP6 = f (CPP28 CPP29 CPP30) |
CPP28 | CMA | DS starting material identity (S.2.3) |
| CPP29 | CMA | DS reagent identity (S.2.3) | |||
| CPP30 | CPP | DS synthetic route (S.2.2) | |||
| CPP5 | Test | DS identity (S.4.1) | |||
| CPP6 | GMP | GMP dispensing (PQS) | |||
| CPP7 | Test | DP identity (P.5.1) | |||
| Assay CQA3 |
CQA3 = f (CPP8 CPP9 CPP10) CPP8 = f (CPP6 CPP28 CPP29 CPP30) |
CPP28 | CMA | DS starting material identity (S.2.3) | |
| CPP29 | CMA | DS reagent identity (S.2.3) | |||
| CPP30 | CPP | DS synthetic route (S.2.2) | |||
| CPP6 | GMP | GMP dispensing (PQS) | |||
| CPP8 | Test | DS assay criteria (S.4.1) | |||
| CPP9 | IPC | Tablet weight (P.3.4) | |||
| CPP10 | Test | DP assay criteria (P.5.1) | |||
| Uniformity of Dose |
Content Uniformity CQA4 |
CQA4 = f (CPP11 CPP12 CPP13 CPP14 CPP15 CPP16) CPP11 = f (CPP31 CPP32 CPP33) |
CPP31 | CPP | DS milling operation (S.2.2) |
| CPP32 | IPC | DS end of milling particle size (S.2.4) | |||
| CPP33 | CPP | DS step 3R drying temperature (S.2.2) | |||
| CPP11 | Test | DS particle size criteria (S.4.1) | |||
| CPP12 | CMA | Excipient grade (P.1) | |||
| CPP13 | CPP | Screen aperture (P.3.3) | |||
| CPP14 | CPP | Blend revolutions (P.3.4) | |||
| CPP15 | CPP | Lubrication number of rotations (P.3.4) | |||
| CPP16 | Test | Content uniformity criteria (P.5.1) | |||
|
In vitro Drug Release |
Dissolution CQA5 |
CPP11 = f (CPP31 CPP32 CPP33) |
CPP31 | CPP | DS milling operation (S.2.2) |
| CPP32 | IPC | DS end of milling particle size (S.2.4) | |||
| CPP33 | CPP | DS step 3R drying temperature (S.2.2) | |||
| CPP11 | Test | DS particle size criteria (S.4.1) | |||
| CPP17 | CPP | DP formulation (P.1) | |||
| CPP18 | GMP | Dispensing (PQS) | |||
| CPP15 | CPP | Lubrication – number of rotations (P.3.4) | |||
| CPP19 | Test | Dissolution criteria (P.5.1) | |||
| Degradants and Impurities/Shelf Life | Degradation
Products CQA6 |
CQA6 = f (CPP20 CPP21 CPP22) CPP21 = f (CPP34 CPP35 CPP36) CPP34 = f (CPP38 CPP39 CPP40 CPP41 CPP42) CPP22 = f (CPP37) |
CPP34 | IPC | Intermediate IPC (S.2.4) |
| CPP37 | CMA | DS reagent, solvent, and material specifications (S.2.3) | |||
| CPP38 | CMA | DS starting material identity and specification (S.2.3) | |||
| CPP39 | CPP | DS step 1 reaction completion IPC (S.2.4) | |||
| CPP40 | CPP | DS step 2 Stoichiometry (S.2.2) | |||
| CPP41 | CPP | DS step 2 reaction temperature (S.2.2) | |||
| CPP42 | IPC | DS step 2 reaction completion IPC (S.2.4) | |||
| CPP35 | CPP | DS step 3 reaction temperature (S.2.2) | |||
| CPP36 | CPP | DS step 3 isolation temperature (S.2.2) | |||
| CPP20 | Test | DS impurity limits (S.4.1) | |||
| CPP21 | Test | DS residual solvents limit (S.4.1) | |||
| CPP22 | Test | DP degradation limits (P.5.1) | |||
|
Water Content CQA7 |
CQA7 = f (CPP23 CPP24 CPP25) | CPP23 | Test | DS water content limit (S.4.1) | |
| CPP24 | CMA | Excipient grade (P.1) | |||
| CPP25 | Test | DP water content limit (P.5.1) | |||
By clearly defining the product control strategy, the MAH demonstrates assurance of manufacturing process control and product quality and substantiates how a science- and risk-based approach delivers appropriate product quality. When the MAH combines this with a robust PQS, they (a) can guarantee “delivery of products with the quality attributes appropriate to meet the needs of patients, health care professionals, regulatory authorities and other internal and external customers, (b) develop and use effective monitoring and control systems for process performance and product quality, thereby providing assurance of continued suitability and capability of processes and (c) identify and implement appropriate product quality improvements, process improvements, variability reduction, innovations and pharmaceutical quality system enhancements, thereby increasing the ability to fulfil quality needs consistently.” 2
Regulatory Binding Elements/ECs
In the proposed comprehensive QOS, a summary of pharmaceutical development information along with a table that conveys the control strategy is provided. This provides substantiation for the regulatory binding elements such as process descriptions, specifications, and test methods for the DP, DS, raw materials, excipients, packaging materials, and components.
If the MAH chooses to use the tools in ICH Q12, then the regulatory binding information will include both ECs and their associated proposed reporting categories. All parameters that have been identified as having a functional relationship with a CQA are categorized as ECs. Process parameters that have been identified as having no functional relationship with a CQA are assigned as non-critical and categorized as supporting information. ECs describe how a company intends to manufacture and control product quality. Risk-based reporting categories for changes may be proposed considering the control strategy. A PLCM3 document provides a listing of ECs and reporting categories as well as other commitments such as stability, change management, and post-approval change management protocols.
Discussion
Pfizer submitted a comprehensive QOS along with an original new drug application (NDA) to the US FDA in early 2018. The summary included the concepts outlined in this article and aimed to clearly convey the product control strategy. Following approval of the NDA, a post-approval feedback meeting was held with the FDA to solicit feedback and discuss the comprehensive QOS as a viable and useful review aid for the assessment the NDA.
The reviewers affirmed that the single summary document was easy to use and provided an overall picture of the product. While the document was quite lengthy, and hyperlinking to the data in Module 3 would have been more efficient than repeating tables of information, the comprehensive QOS was beneficial, especially for communication among the multiple regulatory disciplines that contribute to review of CMC content. During the NDA review, relatively few queries for CMC information were received and the reduction was likely (as least partially) because reviewers from other disciplines were able to quickly look at the comprehensive QOS for broader context without issuing an information request.
Based on learnings from this pilot, an optimized proposal for the content of the comprehensive QOS would include key information with linkage to, rather than repeating information contained within, Module 3. Table 4 shows a proposed outline of the information that should be included in the comprehensive QOS.
| Section within CTD Module 2 | Purpose | Suggested CTD Sections to Summarize | |
| DS | DP | ||
| Target Product Profile (TPP) | Defines the nature of the product, indications (and contraindications) and usage, dosage and administration, dosage form and strengths, targeted patient population, and packaging and storage requirements. | N/A | P.2 |
| Quality Target Product Profile (QTPP) | Describes the elements of the intended DP related to quality, safety, and efficacy and links the CQAs of the DP back through the TPP to the needs of the intended patient population. | S.1 | P.2 |
| Pharmaceutical Development: Risk Assessment and Development of Control Strategy | Identifies quality attributes and process parameters with the potential to impact CQAs and presents resulting risk assessment verified by experiments conducted. |
S.2.5, S.2.6, S.3, S.4.3, S.4.4, S.4.5, S.7.1, S.7.3 |
P.1, P.2, P.3.5, P.4.3, P.4.4, P.4.5, P.4.6, P.5.3, P.5.4, P.5.5, P.5.6, P.8.1, P.8.2, P.8.3, A.1, A.3 |
| Summary of Comprehensive Control Strategy | Provides proposed controls to ensure the acceptance criteria for each DS and DP CQA are met. |
S.2.1, S.2.2, S.2.3, S.2.4, S.4.1, S.4.2, S.5, S.6, S.7.2 |
P.3.1, P.3.2, P.3.3, P.3.4, P.4.1, P.4.2, P.5.1, P.5.2, P.6, P.7, P.8.1, P.8.2, A.2 |
|
Summary of Regulatory Commitments / ECs and the PLCM (if applicable). Also include any PACMPs (if applicable) |
The ECs delineate ‘which elements in the application are considered necessary to assure product quality and therefore would require a regulatory submission if changed post-approval.’ 3 The corresponding PLCM document serves as a central repository for ECs and the associated reporting categories (based on potential risk to quality, i.e., prior approval, notification, not reported) for changes made to ECs. Post Approval Change Management Plan(s) (PACMPs),3 if relevant, provides the requirements and studies needed to implement a future change. |
S.1, S.2.1, S.2.2, S.2.3, S.2.4, S.4.1, S.4.2, S.5, S.6, S.7.1, S.7.2 |
P.1, P.3.1, P.3.2, P.3.3, P.3.4, P.3.5, P.4.1, P.4.2, P.5.1, P.5.2, P.6, P.7, P.8.1, P.8.2, A.2 |
By summarizing the ECs in the comprehensive QOS, the MAH establishes a clear standard for subsequent post-approval change management.
Conclusions
The comprehensive QOS intends to provide a consistent format that could facilitate a better and faster product understanding for both internal and external stakeholders (e.g., inspectors and other multidisciplinary regulatory and industry stakeholders). By clearly integrating and explaining the content of Module 3, the effectiveness of regulatory submissions and reviews (i.e., reduced assessment time and/or queries) could be improved. The comprehensive QOS provides a direct link between the patient and the quality attributes of the product with the safety and efficacy for the patient. It introduces an appropriate science- and risk-based framework to demonstrate how enhanced process knowledge and product understanding effectively manage risks to product quality by establishing a robust control strategy that is maintained through the life cycle of the product.
The ICH M4Q EWG is currently considering options for the structure and format of the Module 2 that provide easy access to data, analysis, and knowledge management. By structuring Module 2 with a format as described in Table 8, the need for customized summary documents could be addressed and the customized documents eliminated. Furthermore, the comprehensive QOS could serve as the starting point for the CMC assessment. This should foster alignment between inspections and assessments by providing the inspector with a clear concise overview of the product, increase consistency in regulatory reviews among different regions globally, and enable mutual reliance and recognition, particularly for unmet medical needs, to expedite patient access of innovative medicines.14
About the Authors
Acknowledgements
The authors would like to acknowledge the contributions of the following colleagues, especially those involved with drafting the original PhRMA white paper and those involved with reviewing the current paper, including Dan Bollinger, Andrew Chang, Xavier Castell, Graham Cook, Frank Diana, Olivier Dirat, Jeff Ferguson, Georges France, Tom Garcia, Betsy Fritschell, John Groskoph, Nirdosh Jagota, Bob Kelly, John Lepore, Rick Lit, Stephen Mason, Moheb Nasr, Sarah Pope Miksinski, Ron Ogilvie, Ken Oh, Mark Rosolowsky, Greg Rullo, Tom Schultz, Steve Tyler, Timothy Watson, Jim Webb, and Diane Zezza.









