Plastic Process Waste in Biopharmaceutical Manufacturing

This article presents a comprehensive analysis of plastic waste generation in the biopharmaceutical industry, focusing on using single-use technologies (SUT) in bioprocessing for monoclonal antibody (mAb) production. It aims to inform sustainable practices within the biopharmaceutical industry and to encourage the development of more sustainable disposable technologies.
Since the 1990s, the biopharmaceutical sector has increasingly adopted single-use systems in manufacturing, raising concerns about the sustainability of these practices due to the resulting plastic waste streams. A commercial bioprocess modeling package estimates the plastic usage for typical mAb production at varying scales, offering a detailed exploration of waste composition, including fluid handling components, filters, and resins.
This batch-related plastics usage is linked to the forecast of worldwide mAb requirements to estimate the total annual metric tons per year of bio-process-related plastic waste generated by the industry. The findings highlight the relative volume of bioprocessing waste compared to medical waste from healthcare facilities, discussing possible synergies to identify recycling and waste treatment options. Through quantitative assessments, this study explores the scale of plastic waste produced, comparing it with the healthcare sector.
Background
Since adopting SUT in biopharmaceutical manufacturing in the 1990s, there has been concern about the generated plastic waste streams. Estimates for plastic waste based on production quantities provide the biopharmaceutical industry with methods to predict the waste produced and to establish disposal methods as SUT products expand into the mainstream production of biologics.
The Challenge
This concern over plastic waste is not new and has grown as the implementation of single-use technologies has expanded.1 Over the years, several assessments have evaluated the comparative environmental benefit of single-use (SU)-based vs. stainless steel (SS)-based manufacturing.2, 3,4 Despite the evidence that SUT in bioprocessing have an overall advantage over SS-based manufacturing in regard to utilities, clean water, steam, and clean-in-place solutions at more minor scales, the concern over the amount of generated plastic waste persists.
To assess the significance of plastic waste generated by bioprocessing, the scale of the plastic waste stream must be determined, allowing its relevance relative to other sectors to be estimated. This article addresses this challenge. In addition, it will classify the waste into broad categories: fluid handling, filters, and resins.
The potential total volume of bioprocessing waste and its relative scale compared to healthcare plastic waste informs the value to recyclers and opportunities to integrate with existing treatment options. It could also provide input for the development of new treatment options.
The approach used to assess the plastic stream is to estimate the plastics used to manufacture a batch of products using a commercial bioprocess modeling package.5 The bioprocess modeling package was used to model the typical mAb manufacturing processes—at 2,000-liter (L) SU and 15,000-L SS scales, described in more detail later. The amount of product manufactured each year by scale and technology was estimated. From an understanding of the market for mAb manufacturing, an estimate of the number of batches and the amount of plastic per batch was made, thus generating the annual total plastic usage.
Product Basis
Referring to data published in late 2023, the analysis by Ecker,6 identified that 85% of the products in development and commercial manufacture are in mammalian cell–culture systems and that mAbs account for 98% of the kilograms of mammalian marketed products. Antibody dose requirements are significantly higher than those for many other product classes, such as enzymes, hormones, or cytokines. Therefore, it is reasonable to assume that mAbs account for the bulk of the manufacturing capacity requirements and, as such, would be a good baseline product class to estimate the plastic waste stream and would be a realistic indication of the scale of biopharmaceutical waste streams.
Market Size and Manufacturing Configuration
This study focuses on commercial manufacturing of mAbs. The first step is to determine the number and size of batches now and in the future, which requires an estimate of current and future demand expressed in kilograms per year (kg/yr). The second step is to estimate the different manufacturing scales and bioreactor types. The last step is to get an idea of the bioreactor titers used in commercial manufacturing.
For this analysis, the demand for all cell-culture products is based on information supplied by Ecker.6 The estimate is that the total mass of mam-malian-derived products in 2023 was 41 metric tons, rising to 94 in 2028. Products related to mAb account for 98% of this tonnage.
Outputs from a manufacturing capacity database7 provided records of historical cell culture installed capacity. This data estimates the proportion of stainless-steel bioreactors’ (SSB) capacity vs. single-use bioreactors’ (SUB) capacity. The proportion of SUBs in 2016 was 7.6%, rising to 12.1% in 2024, and is predicted to increase to 21.3% by 2028.
This analysis used the year-by-year distribution bioreactor capacity between SUBs and SSBs.7 To identify the number of batches manufactured, it was assumed that the typical size of an SUB was 2,000 L, which is historically the average size of SUBs available. Larger sizes are now available and will be adopted over the coming years, reducing the amount of plastics used in scaling up manufacturing.
This analysis compared SSBs to SUBs using a 15,000-L working volume bioreactor size. This is considered a typical batch size for an SSB.8 Assigning a titer to the different products is necessary to approximate the number of batches per year. A simplifying assumption is to use an average titer for all products.
In a paper by Rader,9 the estimate for the average titer of all products on the market in 2020 is 3.5 grams per liter (g/L). This figure is the basis for this analysis. Using typical process parameters for the mAb process, the model calculates the batch size for the purified bulk drug substance at 4.9 kg/batch for the 2000-L bioreactor and 36.9 kg/batch for the 15,000-L bioreactor.
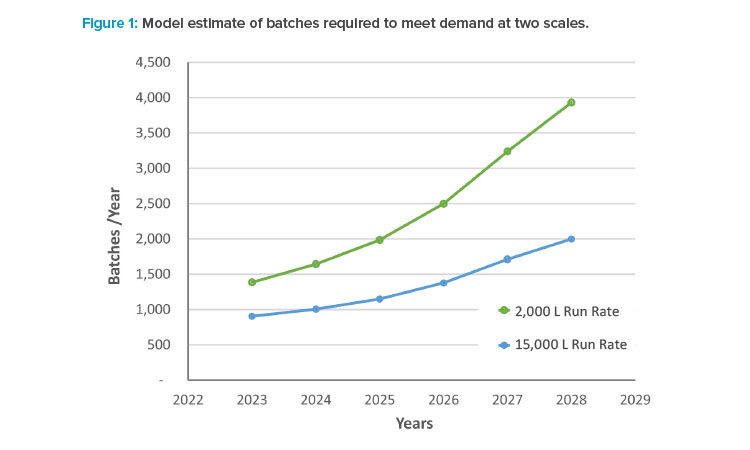
The output of the model is summarized in Figure 1 which shows the distribution of batches per year between the large scale 15,000-L SSB and the smaller scale 2000-L SUB runs. This estimate of manufacturing batches per year when combined with bill of materials data is used to forecast plastics usage. This approach is presented later in this article.
Plastics Generation
Having determined the number of batches at different manufacturing scales, the next step is determining the amount of process plastics required to manufacture a batch of material. The study used a bioprocess modeling package5 for biotechnological and related applications used for tech-no-economic and eco-design analyses. The software package uses process configuration supplied by users that reference a database of commercially relevant resources, including costs and weights for equipment and materials based on averaged supplier data. It was used in this study because it can perform bioprocess modeling and calculate the mass of plastics used to manufacture a product batch. This model has generally been accepted as the default modeling tool by numerous industry forums, end users, and supporting vendors via publications over the past 25 years.
| Configuration | |||
|---|---|---|---|
| 1 | 2 | 3 | |
| Type | SU | SS Only | SS Hybrid |
| Bioreactor Size | 2,000 L | 15,000 L | 15,000 L |
| Batch Size | 4.9 kg | 36.9 kg | 36.9 kg |
| Plastic Use (measured in kg per batch) | |||
| Resins | 4.2 | 28.0 | 28.0 |
| Bags and Tubing | 310.0 | 0.0 | 140.7 |
| Filters | 197.5 | 140.3 | 178.9 |
| Packaging | 255.8 | 84.2 | 173.8 |
| Totals | 767.5 | 252.5 | 521.4 |
| PMI ( measured in gram of material required to manufacture a gram of product) | |||
| Process Water | 3,376 | 2,789 | 2,788 |
| Process Plastics | 156 | 7 | 14 |
| Process Materials | 22 | 24 | 24 |
| Cleaning Water | 0 | 6,768 | 5,434 |
| Cleaning Materials | 0 | 10 | 8 |
| Totals | 3,554 | 9,598 | 8,268 |
PMI, process mass intensity; SS, stainless steel; SU, single use.
Three modules are used to determine the data sets:
- Resource database: a proprietary database of all the “typical” commercially supplied components required for bioprocessing, ranging from small to large scale. It includes plastic components such as bags, tubing sets, and filters. Each element will have a scale, cost, and weight of plastic. This data set is updated regularly based on supplier data that is averaged; the last update was in 2023.
- Recipe module: defines the process. It comprises a set of scaling rules and a scaleless recipe, which, when given a scale, calculates the mass balance, process equipment, process material, labor, and consumable requirements that reference the resource database. The processing module drives the estimate for the utilities, solution management, facility, and energy modules.
- Output module: generates a wide range of outputs. This study uses the bill of materials report, which identifies all the consumable components in terms of the size, number, and mass required to manufacture one batch. This information is used to determine the category and mass of plastics used.
The process used as a basis for mAb manufacture is based on a baseline reference process defined as a part of the Biopharm Manufacturing Technology Roadmap in 2017.10 Adjustments were made to include tubing and plastic fluid transfer sets often deployed in SU manufacturing setups based on a presentation by Goldstein.11 Three different configurations were modeled to estimate the plastics used per batch.
- Configuration 1: This SU facility has a bioreactor capacity of around 2,000 L. Although larger-scale bioreactors are available and showing promise in reducing plastic use overall, they are relatively new and only represent a small proportion of the installed SUB base to date. This will change in the future.
- Configuration 2: A fully SS production line with no SU components. This is based on a 15,000-L bioreactor scale. This is representative of the legacy facilities.
- Configuration 3: A hybrid SS production line with SU components used in the seed train and fluid management where the size fits. This is based on a 15,000-L bioreactor scale and is representative of the newer SS facilities.
Each configuration was set up and modeled, and estimates of plastic use were determined. These are detailed in Table 1, together with the process mass intensity (PMI) values. 12 PMI accounts for all materials used within the process, including cleaning and direct materials, as a ratio of input materials to the mass of the product.
The PMI compares the efficiency of processes in converting the input materials to products. In this case, an SU process uses considerably more plastics per output unit than the two larger-scale SS-based processes. This contrasts with the use of water, where SS-based manufacturing uses significantly more water for cleaning.
There is little published data on plastic use by processes that can be used to confirm the validity of the model predictions. Budzinski13 published a paper on streamlined life cycle assessment of SUT. A comparison was made between the plastics used in the paper and the model’s predicted value. The basis of comparison was PMI for the plastics category, which measures the gram of plastics required to manufacture a gram of product.
The Budzinski paper13 references a 2,000-L SU process using prepacked columns; the PMI for SU components is 513. When the same workflow is modeled in the BioSolve Process,5 the PMI for the plastics is calculated as 507. There is good alignment between the published paper and the Bio-Solve Process model.
The Budzinski paper13 used a titer of 1.07 g/L, which is low compared to commercial titers. The model used for this paper used the same process with an increased titer of 3.5 g/L and reusable chromatography columns. With these changes, the PMI for plastics dropped to 156. This aligns with expectations given that PMI upstream is directly influenced by titer and downstream processing PMI for bags and tubing decreases with increasing scale.
Plastics Use by Category
In this study, by estimating the number of batches for each configuration and knowing the mass of plastic waste generated per batch, the total quantity of plastic waste generated yearly can be calculated for mAb manufacturing. The critical assumption is that the amount of plastic packaging associated with the consumables is estimated to be 50% of the consumable mass. The second assumption is that the proportion of products manufactured in the hybrid SS vs. all SS is again assumed to be 50%.
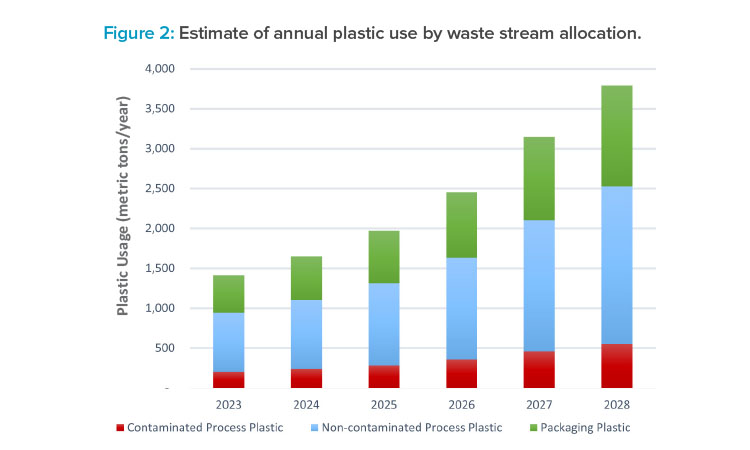
Figure 2 gives the total estimate of annual process plastics use for the next five years. The figures are based on the actual plastic mass of materials going into the process; the waste streams will be heavier than just the calculated plastic waste, as they will also contain process water and solids. The compounded annual growth rate over these five years is 22%.
The plastics are broken into three categories: packaging plastic, contaminated process plastic, and noncontaminated process plastic. Packaging comprises 33% of total plastic waste and can be directly processed from the receiving warehouse, where materials are removed from their primary packaging such as cardboard and bubble wrap. This material avoids contamination, as it is away from the manufacturing and laboratory spaces.
Waste is deemed to be contaminated if it is potentially exposed to cell-culture microorganisms. Of the total plastic waste, 14% is contaminated process plastics, which may need specific decontamination measures before onward processing. Noncontaminated process plastic waste represents 53% of the total plastic waste.
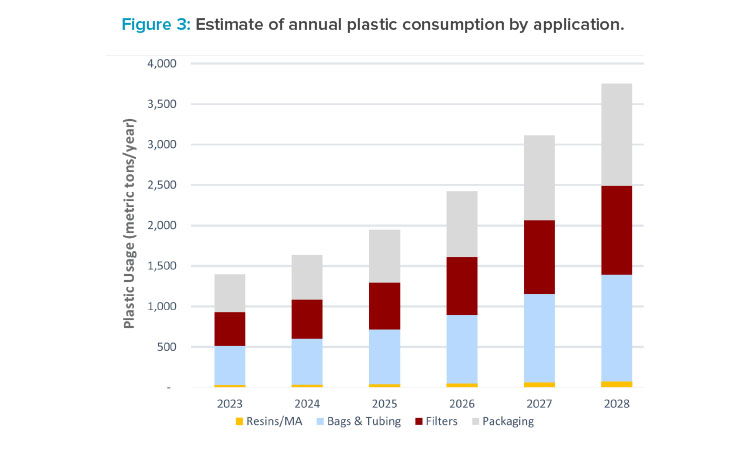
The plastics consumption data can be analyzed in terms of its application in the process. Figure 3 is broken out into four categories, as these categories will influence the onward processing of the waste streams. Ignoring packaging (33%) and resins/membrane adsorbers (MA), the bulk of the plastics used by the process fall into two categories: fluid management, represented by bags and tubing (34%), and filters (29%). The processing of filtration waste (including depth filters, tangential flow filtration, and sterile filters) would be different from fluid management (hold bags, mixer bags, bioreactors, tubing, and connectors). These two main categories would be further segregated into contaminated (biologically) and non-contaminated, with the contaminated waste stream typically requiring pre-treatment at the manufacturing site before onward processing.
Although the exact composition of the plastic products is not readily available, the component weights used are based on averages across different suppliers’ products. On that basis, resins mostly contain silica or cross-linked agarose. Bioprocess container films usually contain polyethylene, polyester, nylon, ethylene vinyl acetate, and ethyl vinyl alcohol layers. Tubing contains silicone, thermoplastic elastomer, and polyvinyl chloride. Filters contain polyethersulfone and polyvinylidene fluoride membranes and a polyethylene housing.
Though not representative of the whole biopharmaceutical sector, mAbs are dominant in terms of manufacturing scale. So, how much more must be added to account for all the other products and drug products? The total amount of plastics will likely be at most two to three times the mAb quantities. On that basis, total consumptions of between 1,500–4,500 metric tons/year (mt/yr) for 2023 and 3,900–11,700 mt/yr by 2028 is a reasonable estimate. By any measure, there is a significant growth in the waste streams. As the industry is focusing on waste reduction and net-zero greenhouse gas emission strategies, this is an issue that needs to be addressed now.
One question is how significant a waste stream is relative to other elements of the supply chain that may share the same challenges and could work together for solutions. It’s an important question because it will influence third-party interest in managing the waste stream vs. suppliers taking on the responsibility of managing waste streams associated with their products.
Plastic Handling in Healthcare
This article has discussed the scale of plastics generated by the biopharmaceutical manufacturing industry, and the economic sector downstream in the supply chain is the healthcare sector. Although different in scale, healthcare facilities use large quantities of SU plastics and have similar challenges in managing these waste streams. As a mature sector, healthcare facilities have established third-party companies that handle their waste streams. In terms of volume, how does the biopharmaceutical industry compare?
Here are two cases: the UK and the US. The UK National Health Service (NHS) has a strategy to reduce clinical waste and collects figures for all the healthcare in the UK in its NHS clinical waste strategy document.14 The NHS healthcare providers produce approximately 156,000 mt/yr (2023) of clinical waste that is either sent to high-temperature incineration or for alternative treatment. It is estimated that 12,700 metric tons of waste are generated daily in US healthcare facilities; about 20%–25% of the waste is plastic.15 This represents about 930,000–1,160,000 mt/yr of plastic waste.
Healthcare facilities have similar challenges in identifying solutions for their plastic waste. These facilities also have a mix of contaminated and non-contaminated materials of various plastic types and logistical hurdles for sorting the waste and identifying recycling solutions.16 Healthcare plastic materials include rigid trays, bottles, flexible packaging, sterilization wraps, and multi-layer bags for solution delivery.
There are many parallels between these challenges and the plastic waste that results from the biopharmaceutical industry. Solutions that may improve the healthcare industry’s plastics waste could also be applied to biopharmaceutical waste. This is also an opportunity to combine bioprocessing waste with healthcare waste to increase the total volume that recyclers should consider as feedstock for their processes.
Working more closely with recyclers to identify or develop solutions for this high-quality plastic waste is critical. Recyclers are continually looking for large-volume streams of plastic to use as feedstock in their processes. They must continuously flow into their facilities to maintain efficient processing and manage costs. Mechanical recycling requires a mono-material or minimal-complexity plastic stream, whereas some advanced recycling technologies being developed can handle more complex material mixes. Sorting technologies continue to evolve to simplify managing incoming material for recycling. Significant variations in the local recycling infrastructure are also available depending on the city, state, or country in question. These considerations also factor into the options available for recycling biopharmaceutical waste.
Conclusion
The results of this study provide an initial estimate of the volume of biopharmaceutical manufacturing plastic waste that may be available for recyclers to consider as feedstock. The study used market information about mAb production and the BioSolve Process5 with its embedded SU bioprocessing database of materials for various production scales. This resulted in a conservative estimate of 1,500–4,500 mt/yr and is poised to continue increasing over the next five years to as much as 3,900─11,700 mt/yr. The total volume would be greater when considering other materials made using SU bioprocessing technologies, but likely not many orders of magnitude.
For comparison, healthcare plastic waste examples were examined. Two other reports for healthcare plastic waste indicate the scale observed there:
- It has been indicated that the UK NHS disposes of 156,000 mt/yr, with only about 5% of that plastic waste being recovered.14
- In the US, 12,700 metric tons of waste are generated daily in healthcare facilities, and 20%–25% is estimated to be plastic waste, approximately 1,000,000 mt/yr.15
The estimated bioprocessing waste, although not at the scale of healthcare waste, also represents a significant volume of plastic that is mainly going to incineration, waste-to-energy recovery, and landfills.17 There is an opportunity to continue working with recyclers to identify alternative solutions at end of use. Given some common challenges with healthcare plastic waste, the biopharmaceutical manufacturing industry could consider collaborating or learning from the healthcare industry to identify recycling solutions.
There are also opportunities for co-located companies to identify solutions to combine their waste volumes to encourage a local recycler to take this material. A single company may need more volume but combining it with others to generate a routine feedstock stream could drive better recycling options.







