For companies focused on producing lifesaving treatments, the positive effects of employee health, well-being, and satisfaction can be easily overlooked, but those positive effects are real. An investment in people results in better research, testing, and manufacturing processes, which leads to more efficient delivery of therapies and treatment to patients worldwide.
Articles should focus on multiple types of management in the pharmaceutical industry. Examples include Project Management, Knowledge Management, Product Management, and Plant/Site Management.
Organizations must continually evolve and adapt in order to grow, sustain, and stay competitive. No organization survives for a long period of time if it does not change with the times. The pace of change is accelerating, and the scale of disruptive market forces is growing by the day.
For companies focused on producing lifesaving treatments, the positive effects of employee health, well-being, and satisfaction can be easily overlooked, but those positive effects are real. An investment in people results in better research, testing, and manufacturing processes, which leads to more efficient delivery of therapies and treatment to patients worldwide.
Organizations must continually evolve and adapt in order to grow, sustain, and stay competitive. No organization survives for a long period of time if it does not change with the times. The pace of change is accelerating, and the scale of disruptive market forces is growing by the day.
Ferdinando E. Aspesi, PhD, is a Senior Partner at Bridge Associates International, LLC, New Jersey, USA, where he advises pharmaceutical companies on quality strategy, organizational design, and quality and compliance issues. In his more than 44 years of experience in the industry, Aspesi has worked in active pharmaceutical ingredient (API) and drug products quality assurance and quality...
Cell and gene therapy (C>) products represent a significant step forward in patient treatment and often offer unique patient benefits. However, product developers face significant hurdles within the regulatory landscape. The tools laid out in the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) Q12 guideline: “Technical and...
The success of the biopharmaceutical industry and the expansion of manufacturing facilities, of both existing companies and newcomers, has put a strain on the number of temporary and permanent skilled workers needed to fill many positions in the Triangle.
The imperative for global action to tackle climate change is clear and the pharmaceutical industry has a key role to play. Governments have entered into international commitments to reduce climate impact (carbon emissions) and protect nature (water, land, air, and biodiversity) with policy frameworks established to facilitate and drive progress against agreed targets.
As the pharmaceutical industry faces ever-changing global challenges and market forces, it must review and revise product design to ensure that quality products remain available in the marketplace while moving toward zero pollution for air, water, and soil. This article provides an introduction on how quality products can integrate sustainability by design.
Although data and knowledge are both stand-alone disciplines that need to be systematically managed, they also must have a connection. Understanding the relationship between data and knowledge management processes and how people are leveraging advances like Pharma 4.0™ combined with these processes enables quality data transition to knowledge that can help pharmaceutical companies. The authors...
As the pharmaceutical industry continues to grow and evolve, a significant contributor to innovation and evolution is mergers and acquisitions (M&A). M&A can enable academic researchers and small companies to fund and commercialize innovative products. In addition, M&A can help larger organizations secure new and complementary technology and products. In the pharmaceutical...
As the industry experiences significant changes to the way we do business, knowledge capture and sharing are more important now than ever before. The maturing digitalization of the biopharma industry’s business and processes is creating an increasingly data- and information-rich environment that requires more effective mechanisms for sharing data and information. The Knowledge Management team...
On 26 January 2022, representatives of the author team for the ISPE Good Practice Guide: Knowledge Management in the Pharmaceutical Industry held a webinar to provide an...
ISPE held an Expert Xchange on 18 January 2022 entitled “Risk-Based Decision Making: Advancing the Integration of Quality Risk Management (QRM) and Knowledge Management (KM).” The session included presentations and interactive exercises that generated new and useful insights into the current effectiveness of the knowledge that flows into QRM and how a knowledge map can be used to diagnose...
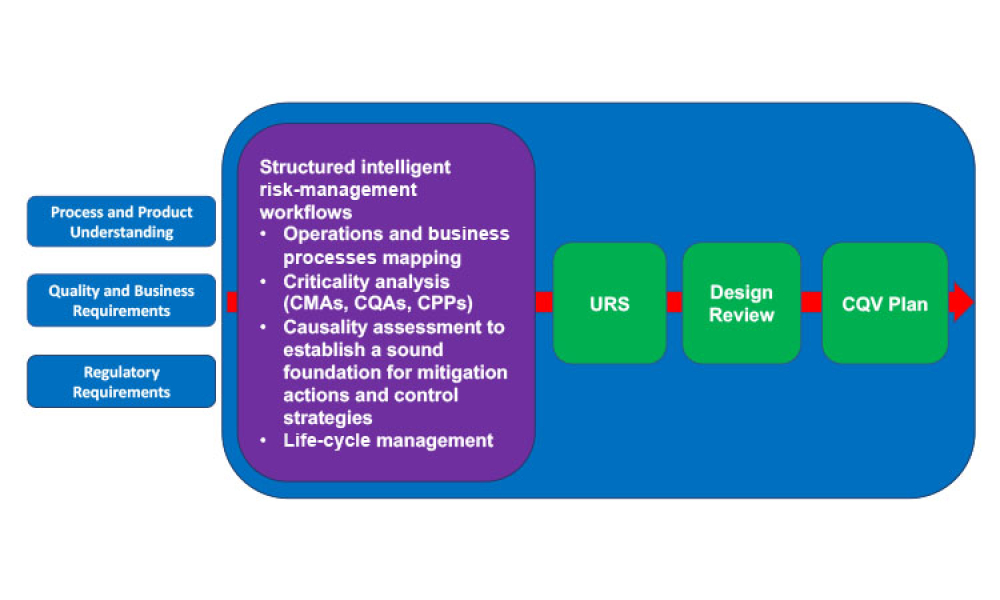
This article revisits the concept of phased engineering, procurement, and construction (EPC) and updates it with risk-based considerations specifically regarding the commissioning, qualification, and validation (CQV) of general life-cycle principles for pharma and biotech projects. Enhancing the relationship between phases of a project, advanced planning, and more formal management of...
This article updates a 2006 Pharmaceutical Engineering® Online Exclusive article titled “Avian Flu—Is My Company Prepared?” by Wendy Haines and Martin Rock.
This article updates a 2006 Pharmaceutical Engineering® Online Exclusive article titled “Avian Flu—Is My Company Prepared?” by Wendy Haines and Martin Rock.
This article updates a 2006 Pharmaceutical Engineering® Online Exclusive article titled “Avian Flu—Is My Company Prepared?” by Wendy Haines and Martin Rock.