Industry Perspective: Bioremediation of Pharmaceuticals

Medical treatments and pharmaceuticals are indispensable in improving quality of life. In recent years, however, pharmaceutical compounds have become a significant group of environmental pollutants, shown to pose risks to human health and have adverse environmental effects.
Pharmaceuticals as Pollutants
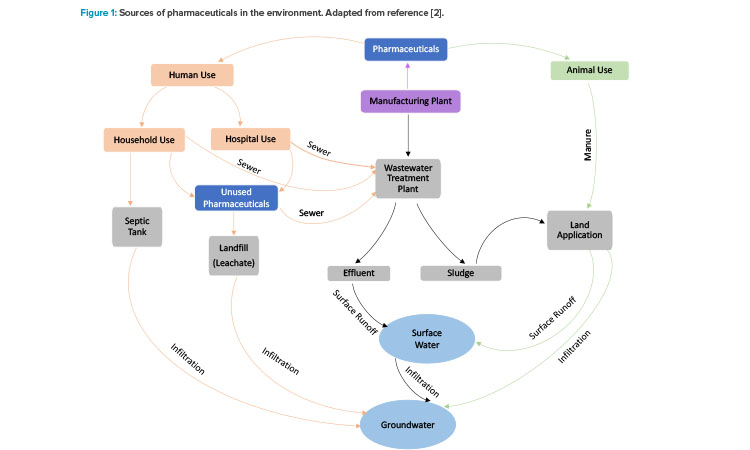
Pharmaceuticals have been detected worldwide in wastewater, surface water, ground water, and soil. North American, Canadian, Japanese, Korean, and European waterbodies contain relatively low amounts (nanograms to micrograms per liter) of various pharmaceutical compounds such as antibiotics, painkillers, hormones, and anti-inflammatory and chemotherapeutic drugs. About 700 different pharmaceuticals were detected in the aquatic ecosystems of 71 countries, according to aus der Beek et al. 1. These compounds enter the environment as byproducts of human and veterinary use through manufacturing waste, human excrement into septic tanks/sewage systems, animal excrement on soil combined with surface or agricultural runoff, household and hospital solid wastes that end up in landfill leachates, and disposal of unused or expired medicine through sewage systems and landfills (Figure 1). 2
Remediation Methods
Among the major contributors of pharmaceutical compounds found in the environment are wastewater treatment plants (WWTPs). Although some compounds (e.g., acetaminophen and caffeine) have been reported to be removed by WWTP processes, most pharmaceuticals reported in the literature are not completely removed by WWTPs, which means they are being discharged into the environment in the treated effluent.
Treatment of pharmaceuticals can be a challenge due to the large quantity, their complex and highly stable chemical structure, and their hazardous nature. Currently available physical and chemical remediation methods—including coagulation/flocculation, filtration, and advanced oxidation processes such as application of ozone, hydrogen peroxide, and ultraviolet light—are not always applicable, can be cost-prohibitive, and may produce secondary pollution. With the increased detection of pharmaceuticals and their metabolites in the environment, the need for more efficient and low-cost remediation technologies such as bioremediation is becoming apparent.
Bioremediation
Bioremediation is the use of naturally occurring or genetically engineered microorganisms (bacteria and fungi) to consume and break down pollutants in contaminated media, including water, soil, and sediment. The US Environmental Protection Agency defines bioremediation as “an engineered technology that modifies environmental conditions (physical, chemical, biochemical, or microbiological) to encourage microorganisms to destroy or detoxify organic and inorganic contaminants in the environment.”3
A well-established technology, bioremediation has been used for decades as an effective method of degrading various forms of chlorinated solvents and petroleum hydrocarbons in groundwater and soil. Bioremediation can be accomplished through natural attenuation, biostimulation, and bioaugmentation in groundwater and soil. Natural attenuation corresponds to the natural remediation capacity of a microbial community present in a contaminated site to achieve contaminant removal. Biostimulation consists of adding nutrients or electron acceptors to encourage indigenous microorganism growth and thus enhance the rate and extent of biodegradation of target contaminants. Bioaugmentation is the inoculation of contaminated sites with strains or microbial consortia (a group of two or more different microbial species that work together) with biodegrading capacities when an appropriate population of microorganisms does not exist or is too slow to stimulate complete remedial of the existing contaminants.
Over the past three decades, bioremediation has been widely studied in environmental biotechnology, and it has been shown that microbial communities in various environments can metabolize a wide variety of chemicals into environmentally acceptable end products. For example, although conventional WWTPs are not designed to remove micropollutants such as pharmaceuticals, the potential for attenuation and degradation of these compounds during the biological treatment processes has been investigated in several studies.2, 4, 5 A manufacturing facility in Arkansas contaminated with chlorinated solvents implemented bioremediation through subsurface injection of emulsified vegetable oil as an electron acceptor (biostimulation) and reduced contamination by more than 90%. Similar bioremediation strategies could be effectively applied to pharmaceutical waste streams and at contaminated sites.
Numerous studies have documented the use of microorganisms and bacterial isolates to break down pharmaceutical waste in WWTPs and in the environment, respectively.6, 7, 8 One study9 evaluated the treatability of bulk drug pharmaceutical wastewater using an activated sludge reactor with acclimatized mixed consortia by integrating with chemical coagulation as the pretreatment process. An 86.6% reduction of chemical oxidation demand (COD) was achieved in pharmaceutical industrial wastewater with the help of the biodegradation process. In another study, modified activated sludge and multistage biofilm processes with microbial consortia involving fungal and bacterial cultures for treatment were found effective in removing toxicity in wastewater from a pharmaceutical company in Sweden.10 Additional research, especially on the isolation and characterization of pure cultures for the degradation of pharmaceutical compounds, could provide the necessary insights to enhance the effectiveness of bioremediation of these compounds.
It is essential to understand the biological transformation of pharmaceuticals and determine the biological mechanisms and degradation pathways responsible for removal to accurately track the ultimate environmental fate of these compounds, and hopefully lead to improved removal of them. Significant progress has been made in understanding the role of microbial metabolism in the transformation and removal of pharmaceuticals in WWTPs and other aquatic systems.
Challenges
Although bioremediation could be a cost-effective technology for removing pharmaceutical waste, biodegradation of pharmaceutical compounds can be challenging, given their diverse and complex chemical structures and relatively low environmental concentrations. Some pharmaceutical compounds, such as ibuprofen, are readily biodegradable, whereas others, such as carbamazepine and trimethoprim, tend to be recalcitrant. Furthermore, biological treatment methods can be sensitive to changes in environmental conditions such as pH, temperature, oxygen, and nutrient levels, as well as sudden changes in the influent’s toxicity levels. These conditions must be optimized and monitored carefully during any bioremediation operation because the treatment’s efficiency directly depends on their stability. An uncontrolled environment may result in the transformation of the pharmaceutical compounds into harmful end products.
Green Approaches
Pharmaceutical waste management can also be improved by using “green chemistry” or “green pharmacy” approaches in which the production routes and entire life cycle of a product are monitored to make them as sustainable as possible by requiring less energy and material, producing fewer undesirable byproducts, or making byproducts easily biodegradable. Many recalcitrant pharmaceutical compounds can now be developed into more biodegradable forms using biologically derived catalysts. Biocatalysts are isolated enzymes and microorganisms that are used as catalysts to produce pharmacologically valuable materials (biopharmaceuticals). Using biocatalysts is not only environmentally beneficial but also cost-effective and therefore more sustainable than using synthetic catalysts.
Conclusion
Ultimately, the pharmaceutical industry and those responsible for risk management would be wise to explore bioremeditation strategies to address pharmaceutical contamination in the environment. As the provided examples highlight, bioremediation offers significant advantages and a cost-effective approach, especially when working to remediate contamination at current and former manufacturing sites.




