Streamlining the Vein-to-Vein Process: The Future of Automated Cell Therapy Manufacturing

Cell therapies, especially autologous chimeric antigen receptor T cell (CAR T cell) treatments, are transforming personalized medicine, bringing new hope to patients with conditions once thought untreatable. However, the manufacturing processes for these therapies remain predominantly manual, presenting significant challenges in scalability, consistency, and making these treatments more widely available.
Manual manufacturing processes are labor-intensive and time-consuming, often involving up to 50 manual steps per dose and requiring approximately 80 hours of labor.1, 2 This complexity not only increases the risk of human error and contamination but also contributes to substantial production costs, with labor accounting for nearly half of the total manufacturing expenses.1, 2
The stakes in cell therapy manufacturing are incredibly high. Lengthy production times not only delay treatment but, in some cases, have devastating consequences. Reports indicate that approximately 20% of patients die while waiting for CAR T cell therapy so there is an urgent need for more efficient processes.3 Research analyzing the impact of reducing waiting times from 1 to 9 months to zero using a health system-level discrete event simulation model suggests that reducing wait times by just two months could improve treatment efficacy by as much as 14%, offering hope for better patient outcomes.4
To tackle these challenges, the industry is rapidly embracing automated and closed manufacturing systems that promise to enhance reproducibility and reduce labor costs through optimized processes. This will open doors for cell and gene therapies to move from niche treatments to first-line options, considering other solutions related to control over side effects. The integration of automated manufacturing, automated data collection, and advanced analytics is not just a step forward, it is important to overcome current manufacturing bottlenecks and enhancing process understanding. This article will provide insights into what a fully automated manufacturing process looks like, the type of data collected, how advanced data analytics tools can support process understanding, and special considerations for validating a fully automated manufacturing platform.
Automated Car T Cell Therapy Manufacturing Platform
The journey of CAR T cell therapy, from extracting a patient’s cells to delivering a life-saving treatment, is often referred to as the “vein-to-vein” process. This personalized therapy has immense potential, but the complexity, variability, and cost of manufacturing present significant hurdles. Automation is revolutionizing this process, streamlining each step to enhance efficiency, ensure consistency, and accelerate delivery. The vein-to-vein process in CAR T cell manufacturing involves several critical stages, each improved by automation.
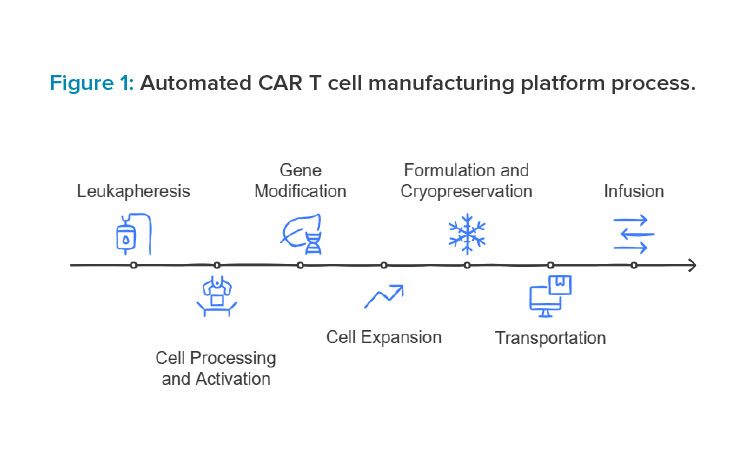
The process begins with leukapheresis, where automated machines ensure precise collection and separation of T cells and digital systems standardize protocols to minimize errors. During cell processing and activation, closed systems and robotic tools handle cell washing, concentration, and reagent addition, reducing contamination risks. In the gene modification step, automated platforms deliver genetic material consistently, with sensors monitoring conditions in real time. For cell expansion, closed bioreactors equipped with sensors and automated media exchange systems support optimal growth.
During formulation and cryopreservation, robotics ensure accurate dosing and controlled freezing for safe storage. Automation also supports transportation and chain of custody with real-time tracking and secure data systems like blockchain to maintain integrity. Finally, at infusion, though less automated, digital tools ensure seamless data transfer to clinicians for patient care. Automation across these stages improves efficiency, consistency, and safety in CAR T cell manufacturing.
Platform Design for Automated Car T Cell Manufacturing
The automated manufacturing platform is designed with modular workstations that seamlessly handle the entire process, from leukapheresis to cell expansion and final infusion. Each workstation integrates specialized automated instruments—including cell separators, bioreactors, and cryopreservation units—to streamline and standardize the workflow. Instruments are interconnected through a centralized software system that orchestrates the entire process. This system manages workflows, tracks patient-specific samples, and provides real-time data monitoring.
By linking all components, the platform reduces manual intervention, minimizes errors, increases efficiency, and ensures compliance with strict regulatory requirements. Advanced sensors and analytics tools are embedded within the platform to monitor critical parameters such as temperature, pH, and cell viability at every stage. This real-time monitoring ensures optimal conditions are maintained, enhancing product quality and consistency.
Automated Data Collection for Advancing Cellular Therapy Manufacturing Processes
As the advanced therapy medicinal products (ATMPs) industry matures, the need for a standardized approach for process characterization becomes more apparent. Lack of process characterization can lead to risk of batch failure, higher costs, and impaired quality, which are especially critical in personalized ATMP products. Process characterization is paramount to tech transfer, e.g., between sponsor and contract development and manufacturing organizations (CDMOs) or different facilities. The nature of personalized ATMPs has long been suggested to be ideal for decentralized manufacturing, which requires a mature, well-characterized process.
Reports indicate that approximately 20% of patients die while waiting for CAR T cell therapy so there is an urgent need for more efficient processes.
The complexity of ATMP processes, with many intervention steps and interdependent process parameters, requires solutions for process control and final product testing. Furthermore, the necessity for manual processes can lead to operational inefficiency. The inefficiencies increase as the process and individual steps are more disconnected. The vein-to-vein process in personalized ATMPs includes not only the actual manufacturing process as in more traditional pharmaceutical and biotechnology manufacturing, but this process starts and ends at the treatment center so it includes logistics and transport to and from the manufacturing site.
Compared to a classic biotechnology process, ATMP manufacturers are facing a multi-step process with additional stakeholders. Moreover, the vast majority of manufacturing processes as of today include equipment with various levels of integration and/or automation capabilities. Although the development of equipment that covers a larger part of the manufacturing process has significantly improved in recent years, the industry currently displays a mixture of automation and manual processes with different levels of automation, but always containing manual intervention and data collection. Even the most mature automation equipment requires manual intervention, e.g., in process analytics and activities at the treatment center.
Another level of diversity in the process is the equipment itself. Although recent developments have reinforced the integration of equipment by providing standard interface capabilities like open platform communications (OPC), many industry-standard devices still lack integration capabilities. This situation leads to disconnected processes and data silos, resulting in data that cannot be used over different process steps for process characterization, as well as a lack of data availability for digital solutions. Moreover, the strict requirements for traceability and process monitoring cannot be compromised, which leads to additional effort to ensure that all requirements are met.
By utilizing advanced instrumentation and continuous monitoring systems, manufacturers can gather high-resolution data in real time across critical stages of production.
Automated data collection is essential for modernizing cell therapy manufacturing. By utilizing advanced instrumentation and continuous monitoring systems, manufacturers can gather high-resolution data in real time across critical stages of production. This data provides valuable insights into cell behavior, enabling tighter control of manufacturing processes and enhancing product quality. Crucial data sources include:
- Flow cytometers: These instruments enable high-throughput characterization of cellular populations by surface marker expression. This capability is crucial for ensuring product identity, potency, and consistency.
- Real-time cell analyzers: These systems allow continuous monitoring of cell growth, viability, and morphological changes, enabling prompt adjustments to culture conditions.
- Next-generation sequencing (NGS): These platforms provide comprehensive insights into genetic modifications and integrity, aiding in the validation of gene-edited or transduced cell products.
- Bioreactors: These automated devices offer data on crucial parameters (cell density, nutrient consumption, and metabolite production), promoting a more controlled expansion environment.
Types of Data and Parameters Necessary for Process Control
To effectively integrate data analytics into cell therapy manufacturing, it is vital to identify the relevant data types and parameters that drive process control. These include:
- Cell phenotype data: Detailed information on surface marker expression, crucial for verifying cellular identity and ensuring product consistency.
- Viability and growth metrics: Continuous monitoring of cell expansion and health ensures optimal culture conditions and product yield.
- Genetic and metabolic data: NGS and metabolic profiling enable confirmation of genetic integrity and optimization of nutrient availability, respectively.
In addition to these core systems, emerging technologies further enhance data collection and analytics. Collectively, these technologies enhance manufacturing oversight by delivering near-real-time insights on cellular metabolism, viability, and genetic status. Commonly tracked parameters include cell density, nutrient levels (e.g., glucose, amino acids), metabolic byproducts (e.g., lactate, ammonia), and surface marker expression. Together, this data guides process adjustments and maintains product quality.
Live Cell Imaging and Analysis
Platforms like CytoSMART and those from Nexcelom Bioscience and IPRASENSE enable real-time monitoring of cell behavior, morphology, and viability. Halo Labs and LumaCyte provide fluorescence microscopy and label-free sorting for enhanced quality assessment.
Flow Cytometry and Phenotypic Analysis
Accellix offers cartridge-based solutions for streamlined phenotypic analysis, and Progen Biotechnik provides lateral flow tests for detecting adeno-associated virus (AAV) capsids.
Genetic, Protein, and Metabolic Characterization
NanoTemper Technologies facilitates multiparameter protein and virus assessment, Parse Biosciences enables single-cell transcriptomics, and DiaMonTech offers real-time glucose monitoring via mid-infrared sensors.
Advanced Analysis and Screening Tools
Myriade Lab and Refeyn specialize in interferometric microscopy for nanoparticle analysis, Sphere Fluidics leverages picodroplet technology for high-throughput single-cell screening, and Megadalton Solutions employs charge detection mass spectrometry for biomolecule characterization.
Integrated Process Monitoring and Control
Unchained Labs and PendoTECH provide versatile tools for cell and gene therapy preparation, real-time process monitoring, and data collection to streamline manufacturing workflows.
Critical Process Parameters (CPPs) and Critical Quality Attributes (CQAS)
Establishing well-defined CPPs and CQAs is essential for achieving reproducible and high-quality cell therapy products: CPPs include environmental factors (temperature, pH, and agitation speed in bioreactors) and transfection or transduction efficiency in gene-modified therapies. CQAs include potency, identity, and purity of the final product and safety indicators, including sterility and absence of process-related contaminants. Careful monitoring and control of these parameters and attributes directly influence therapeutic efficacy, patient safety, and regulatory compliance.
Integration of Advanced Analytical and Support Tools
Advanced analytical tools offer opportunities to refine process monitoring and decision-making. High-content imaging systems provide detailed morphological data that informs on cell health and phenotype. Mass spectrometry enables in-depth proteomic and metabolomic studies, supporting the optimization of growth conditions. Automated liquid handlers standardize sample handling and increase throughput, mitigating variability from manual processes. By merging these analytical capabilities with automated data collection, manufacturers can maintain tighter control of the production process.5
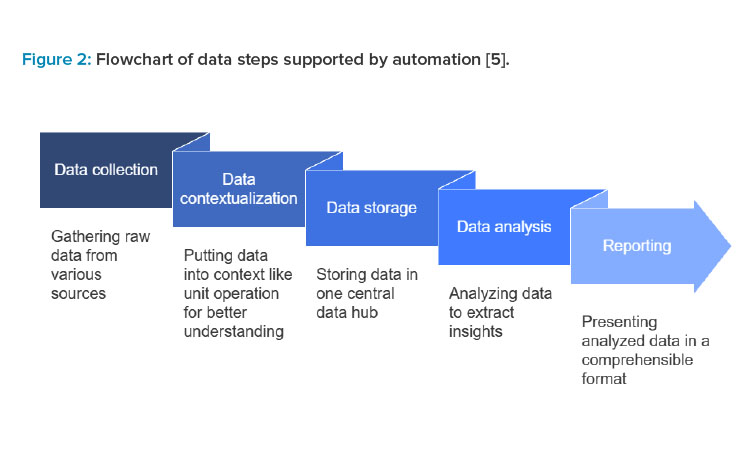
Automated Data Analytics
Automated manufacturing systems equipped with robust data analytics present a transformative approach to addressing current limitations in cell therapy production. Inline and online monitoring of continuous real-time data allows rapid response to process deviations, reducing manual interventions and associated error. Machine learning algorithms and AI-based models can detect patterns, predict performance outcomes, and recommend process optimizations. Automated, advanced bioreactor systems dynamically adjust culture conditions to minimize batch-to-batch variability. Such integrative solutions not only foster consistency but also expedite scale-up efforts, making cell therapies more broadly accessible.
To approach process characterization in such a complex environment, digital ecosystems should be optimized for the specifics in personalized ATMPs. Most ATMP manufacturers use an ecosystem of enterprise resource planning (ERP), patient orchestration, a manufacturing execution system (MES)/electronic batch records (EBRs), and a laboratory information management system (LIMS). However, to support the complex data flow as efficiently as possible, it is useful to optimize the data flow as early in the product life cycle as preclinical or the start of clinical trials.
An optimized data flow and data management concept can drastically speed up the process development, as the data can be used in a more powerful system. Because most manufacturers do not currently have a complete ecosystem in place, the data management concept can be developed on a hybrid ecosystem of manual and digital data and then optimized with the implementation of additional software. The data management concept should be based on process orchestration, including the process orchestration concept, mapping and coordination of the data flow, scheduling components, patient traceability, and equipment coordination.
A central system should oversee the complete process. This central system can be a manually managed spreadsheet—an MES/EBR or a process control system (PCS) system. The centralized system should be optimized for the selected equipment for first implementation, taking any future changes into consideration, including the possibility of future integrations as well as all required data layers. A centralized data hub should be established as early as possible, where all data is collected from different processes, different stages of the product life cycle, and over different facilities. Cloud-based platforms enable real-time data access and provide the required flexibility to the data flow. However, a risk-based approach should be taken to ensure data safety and integrity.
Non-integrated systems and a hybrid landscape of manual and digital data transfer require data bridges, which can be a combination of middleware solutions and manual data transfer. Due to the associated effort, manual data transfer should be optimized and focused on data that provides the most value to the process. Wherever possible, standardized protocols should be used to increase efficiency.
One of the biggest challenges lies in inconsistent data formats and variable data sources. A well-designed concept for data contextualization is required to enable aggregation of information over the complete process and to allow for powerful data analysis. Establishing a data hub with powerful information about the process provides a valuable resource, which is paramount for any sophisticated method for process characterization, like digital twins and the usage of artificial intelligence and machine learning methods.
Validation Considerations for a Fully Automated Manufacturing Platform
Fully integrated platforms used in manufacturing of cell therapies combine multiple software and hardware components—such as an MES, a LIMS, and analytics tools—into a unified ecosystem where data flows seamlessly without manual intervention. This integration must be validated to demonstrate that the platform consistently produces high-quality products.
Validation is defined as the documented process of demonstrating that a system, process, method, or piece of equipment consistently operates according to predetermined specifications and delivers results that meet quality standards. For the purpose of this article, the validation considerations will be focused on computer systems. Compliance with US Food and Drug Administration (FDA)regulations and guidance (e.g., 21 CFR Part 11) ensures that the systems function reliably and maintain data integrity throughout the manufacturing process.
For computer system validation (CSV) of automated cell therapy platforms, some considerations are listed in the next section related to system interoperability, data flow, testing, audit trails, security and access controls, and change management.
System Interoperability and Data Flow Mapping
System interoperability ensures seamless integration of components like the MES, the LIMS, and analytics tools. The MES must automatically retrieve batch records from the LIMS and send real-time data to analytics software for processing without manual intervention. For example, interface testing validates that communication between automated cell culture instruments and the MES is accurate and synchronized.
Understanding how data flows within the platform will ensure secure data transfers and data integrity. By creating a comprehensive data flow map for the platform, stakeholders can visually see where data originates, how it is processed, and where it ultimately resides within the platform. For example, cell viability data from automated microscopy must reliably transfer to the MES for tracking and analysis without corruption.
Testing
Testing the platform under real-world and peak operational conditions will ensure that the overall platform functions as intended. For example, stress testing the MES ensures it can handle a high volume of data when processing multiple patient batches simultaneously, maintaining system responsiveness and data accuracy during peak operations.
Traceability and Audit Trails
Robust audit trails ensure every action, event, and data modification is logged. For instance, any adjustment to temperature or gas levels in bioreactors must be recorded with a time stamp and user details in the MES. These tamper-proof records should be readily accessible for inspections or regulatory reviews. As many industry standard devices in ATMP come with limitations in supporting traceability, a holistic end-to-end process approach is essential. This includes a comprehensive audit trail, chain of identity, and chain of custody, integrating both digital solutions and manual interventions to ensure compliance with regulatory guidelines.
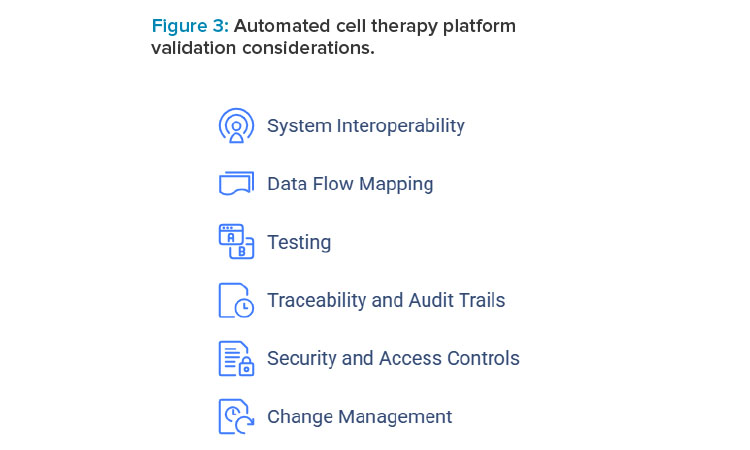
Security and Access Controls
Security and access control validation protects sensitive data and limits unauthorized access. For instance, QC analysts should have permissions to modify analytical test data in the LIMS, whereas operators should be restricted to viewing data, ensuring secure role-based access.
Change Management
Change management ensures the validated state is maintained during system updates or modifications. For example, when upgrading MES software, testing must confirm compatibility with automation hardware and analytics tools before deployment, preventing disruptions in data communication.
Current Challenges and Limitations
Despite rapid advances, several obstacles remain in implementing automation and data analytics.
- Data integration: Merging datasets from multiple sources and instruments poses technical and logistical complexities.
- Standardization: Divergent protocols and parameter definitions hinder cross-site and cross-platform process comparability.
- Regulatory compliance: Automated systems must meet stringent data integrity and traceability requirements, necessitating significant investment in infrastructure.
- Cost: High upfront expenses for automation equipment and software can be prohibitive, especially for smaller organizations.
- Development time: The development time of ATMP processes is large due to limited starting material, process complexity, and incubation times.
The high cost of automation technology limits accessibility, and customizing processes for each patient increases complexity. Overcoming these hurdles requires collaboration among industry stakeholders, regulatory bodies, and academic institutions.
Future Directions and Innovations
Anticipated developments in automation and data analytics are poised to further transform the cell therapy landscape. Blockchain for data integrity supports tamper-proof records, which will bolster traceability from cell harvest to patient infusion. This also enables seamless orchestration with healthcare providers for secure data sharing and coordinated patient care. Edge computing supports local data processing in manufacturing settings, which will enable faster decision-making and increase network resilience.
Advanced AI applications provide next-generation algorithms that could predict potential process deviations and proactively suggest corrective measures, streamlining production and enhancing quality. Emerging advancements in robotics, artificial intelligence, and machine learning are expected to make automated platforms more flexible and scalable. These innovations will address current challenges, improve process efficiency, and enable more cost-effective production, paving the way for broader access to CAR T cell therapies.
Conclusion
Implementing automated systems can streamline validation efforts by providing real-time monitoring, data-driven insights, and enhanced reproducibility. Automation also addresses key process validation challenges by standardizing workflows, minimizing operator variability, and enabling robust data collection for characterization. By integrating automation, manufacturers can improve process control and increase efficiencies to meet validation requirements.
In summary, the integration of automation and data analytics into cell therapy manufacturing offers immense opportunities for refining process understanding, consistency, and scalability. As data collection and analysis technologies continue to advance, industry stakeholders, regulatory agencies, and academic researchers alike must work together to address remaining challenges. Through collaborative efforts, cell therapies can be developed and produced more efficiently, ultimately increasing patient access to these potentially life-saving treatments.
The content in this article is solely the responsibility of the authors and does not necessarily represent the official views of Cellares Corporation.



