"How to Pitch and Shape a Pharma 4.0™ Project” Workshop

During the ISPE Pharma 4.0™ Virtual Conference, the Management Communication working group of the ISPE Pharma 4.0™ Special Interest Group (SIG) held a workshop to support ISPE members in pitching, shaping, and presenting a Pharma 4.0™ project/program to company management.
The workshop, which was organized and led by Davide Smaldone, Corporate IT Demand Manager at Menarini Group, Edoardo Schiraldi, Corporate R&D Business Solutions Specialist at Menarini Group, and Teresa Minero, Founder & CEO at LifeBee–Digitalizing Life Sciences, was very interactive, with 30 attendees from many countries and roles.
The presenters introduced the workshop by sharing their “Management Pharma 4.0™” draft presentation. The target of this presentation is senior management, not technical professionals. A new creative graphic design, sponsored by the ISPE Italy Affiliate, was used to communicate the messages. Smaldone asked the workshop participants for their analyses and comments.
Minero next discussed the values of a 4.0 program both in pharma and in other industries. She summarized the results from a Politecnico di Milano (Italy) research project1 that sought to measure the value of 4.0 in terms of objective parameters. The researchers conducted a survey of more than 4,700 companies from several industry sectors, including 72 companies that were “particularly active” in Industry 4.0 programs from the early 2010s. The analyzed parameters showed that for those companies engaged in 4.0 projects, revenue growth was greater than 8%, gross profit margins increased by more than 37%, and labor-added value increased 60% with an increase of only 31% in labor costs. The research clearly demonstrated the value of the 4.0 mindset and projects for companies on a medium- to long-term scale.
Design Thinking Methodology
Schiraldi kicked off the interactive part of the workshop by introducing the phases of the “design thinking” approach that would be used during the session.
- Empathy phase: This phase aims to identify the possible objections and obstacles, clearly stated or even only perceived, that may prevent stakeholders at a company management level from deciding to approve a Pharma 4.0™ program. The objective is to see the 4.0 proposal through the eyes of the interlocutor (senior manager), through the four dimensions of:
- Hearing—what they hear on the subject from peers, the media, advocates, and others
- Thinking and feeling—what their beliefs, inspirations, and worries are
- Seeing—what they observe and note from their environment, market, product, and processes
- Saying and doing—their attitudes in their usual activity and behavior
- Reframing phase: This phase has two parts.
- Challenges—Reframing begins by focusing on what senior management will identify as the challenges of a Pharma 4.0™ program, as discerned from the empathy phase, and drafting appropriate responses.
- Solutions—Once challenges are identified, it is possible to focus on practical and methodological measures to enable a Pharma 4.0™ program.
To illustrate these phases, the attendees were asked to imagine being one of their company’s senior managers, such as the industrial operations vice president, who has the authority to review and approve a key Pharma 4.0™ program. From this perspective, attendees identified the likely obstacles and doubts that would need remedies and answers before the vice president would let the program proceed, and worked through reframing the challenges to highlight their solutions.
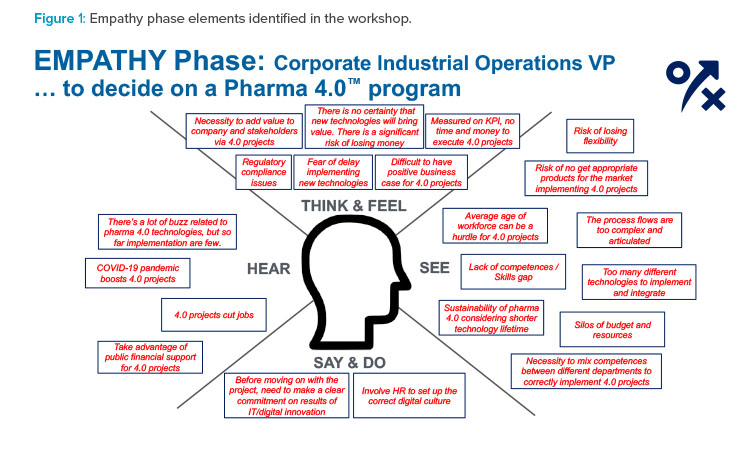
Empathy Phase
Figure 1 shows what the workshop attendees imagined during the empathy phase about the vice president’s perspective on the Pharma 4.0™ potential project. The “obstacles” to the project were clustered as follows:
- Compliance: Obstacles include the vice president’s perception of the company’s current position and their concerns about the need to align the new 4.0 approach with regulatory guidance and the expectations of regulatory agencies.
- Economics: The vice president will be concerned about the costs of a 4.0 program relative to the tangible value that such a program can bring to the company. The vice president may only have a general awareness of the costs of 4.0 programs and requires a well-developed, focused business case for the specific project.
- Knowledge: A wide spectrum of information must be shared with management and personnel. They will require a reliable image of the proposed state-of-the-art 4.0 initiative, including its actual effect on the workforce profile, the project’s features and objectives, its operational aspects, and its impacts on processes.
- Organization: The company’s organization will need to change to benefit from and manage the 4.0 perspective. Success of the 4.0 program will depend on an “all for one and one for all” attitude and willingness to break (or reshape) existing silos.
- Competencies: The vice president will have concerns about workforce 4.0 features and workers’ potential resistance to change. It will be important to show how existing or new structured programs of education and training can fit into 4.0 project implementation.
- Strategy: In recent years, many companies have developed 4.0 pilots and projects, but many of these efforts lack a mid- or long-term strategy with a precise definition of business targets and a sound road map to enable the transformation.
Amid these obstacles, workshop attendees also identified factors that may encourage the vice president to support implementation of a 4.0 company strategy:
- The company may be able to take advantage of public financial support for 4.0 projects (several nations are sponsoring the 4.0 transformation in the industry with special taxation or financial benefits).
- The COVID-19 pandemic has demonstrated the need for and value of 4.0 projects (e.g., programs enabling remote work by digitalizing processes and information).
Reframing Phase
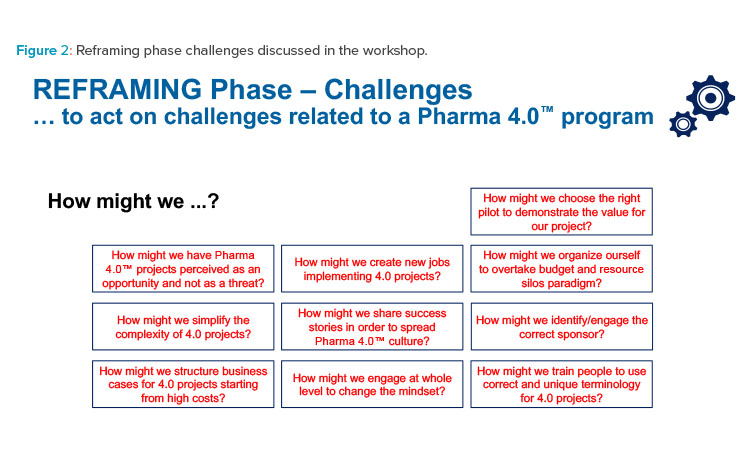
Challenges
Challenges in the reframing phase are certain. However, the Pharma 4.0™ Special Interest Group’s communication framework offers support to overcome them.
Identifying challenges can be a first step toward defining feasible, proactive responses to objections and obstacles arising from the company mindset and organizational structures. Asking “How might we...?” helps define these responses (see Figure 2 for examples).
Workshop participants identified the following main challenges for a Pharma 4.0™ project along with their possible answers:
- Economic challenges: These challenges are resolved mainly through tangible responses to tangible questions.
- Knowledge-related challenges: These challenges are always best managed with tangible, company-focused answers, which can be derived from the general 4.0 knowledge developed through the ISPE Pharma 4.0™ Special Interest Group’s work.
- Organizational challenges: These challenges are mainly addressed by focusing on the company’s self-awareness, the maturity and experience of the company with regard to 4.0 programs, and the ISPE Pharma 4.0™ Special Interest Group’s work in this area.

Solutions
In the reframing phase, solutions are intended as a first set of directions to be pursued to successfully create the conditions for a Pharma 4.0™ project. Solutions proposed in the workshop (Figure 3) can be grouped in the following main areas:
- Design: The main way to answer to organizational, knowledge-related, and economic challenges is to ensure that when the 4.0 initiative project is put into practice, program/project setup, design, and implementation are all robust.
- Knowledge: A successful 4.0 effort will include explicit actions to appropriately increase, widen, and/or consolidate the company’s culture and mindset.
Conclusion
Pharma 4.0™ is a journey the pharmaceutical industry should embrace, and quickly, for the sake of all stakeholders. There’s a lot of value to be achieved for companies, but also some challenges to be faced. Here are five key actions that senior management should put in place to enable success:
- Value the wide range of benefits of Pharma 4.0™ based on the company’s specific business goals.
- Promote the creation of a sound road map, taking into account the digital maturity and the compliance needs.
- Offer sponsorship to drive innovation and open new factual horizons.
- Foster the cultural change necessary to explore trails that have never been walked for an effective transformation.
- Sustain the knowledge sharing among all functions and stakeholders. Professionals need to learn, compare, and debate ideas, successes, and failures with their peers from the whole ecosystem: industry, vendors, academy, and regulators.
During the 90-minute workshop, attendees generated a large volume of challenges and solutions; however, their ideas are not an exhaustive list. If you have identified any touchpoints that we have missed or have any other questions, suggestions, or ideas, please let us know by contacting the chairs of the working team: Teresa Minero and Davide Smaldone.
ISPE and its working groups provide a unique and powerful platform to support managers and professionals in their journey to Pharma 4.0™—only together we will create enough fuel for the thrilling future that awaits our industry.
Acknowledgments
Thank you to everyone who participated in the workshop and to the ISPE staff who helped us create this fantastic interactive event, despite the pandemic.



