Unlocking ATMPs: Reducing Cost as an Obstacle to Patient Access

Advanced therapy medicinal products (ATMPs) have the potential to treat life-threatening, incurable conditions. But access to these therapies remains challenging due to the nature of current ATMP manufacturing models. This article explores solutions, focusing on standardized processing and shared knowledge as gateways to automated, robotic manufacturing and decentralized production.
Background
Using engineered cells, genes, and tissues, ATMPs have the potential to precisely target the root cause of cancers, rare diseases, and other previously incurable conditions. They are distinct from traditional biologics in several ways,1 notably by their reliance on living source material derived from a donor, or, in the case of personalized therapies, from a patient’s own cells.
Life science manufacturers worldwide are drawn to the curative potential of these therapies. A 2023 survey of more than 500 manufacturers showed that 83% have cell therapies in their pipeline.2 Gene therapy programs are attracting significant investment and research, with several advancing from preclinical research to commercial launch.3
To unlock the full promise of these innovative therapies, manufacturers, payers, and government agencies must address the issue of patient access, particularly from the perspective of cost control. Policy reform and innovative payment models are part of the solution.4 Regulations designed to streamline the clinical trial process would help lower cost, for example. Concepts such as innovative contracting—in which payers and manufacturers agree to outcomes-based reimbursement or other models meant to address uncertainty—could also improve patient access.5
Further upstream, collaborations between drug manufacturers, equipment vendors, regulators, hospitals, and academic and public institutions are driving new approaches to ATMP manufacturing—approaches that open the door to standardized processes, simpler facilities, and less costly manufacturing models. Decentralized systems, for example, could put ATMP production units close to a patient’s bedside, facilitating a strong knowledge-sharing network and eliminating the complex supply chains that impede rapid, cost-effective delivery. Decentralization is already in practice in some markets and could transform the future of manufacturing for certain ATMP modalities.6 To reach that future, ATMP manufacturers must document today’s challenges. From that foundational understanding, collaborators across the industry can lay the groundwork for emerging solutions and practices that will bring much-needed therapies to patients.
Meeting Current Manufacturing Challenges
The term “vein-to-vein” often appears in the context of personalized medicine, referring to the manufacturing life cycle that typically begins with the collection of patient or donor cells and ends with the bedside administration of a final therapy. Between those “veins” lies a lengthy process (see Figure 1). Simplifying that process is the key to improved patient access. To do that, one thing is necessary above all: an ecosystem of shared knowledge.
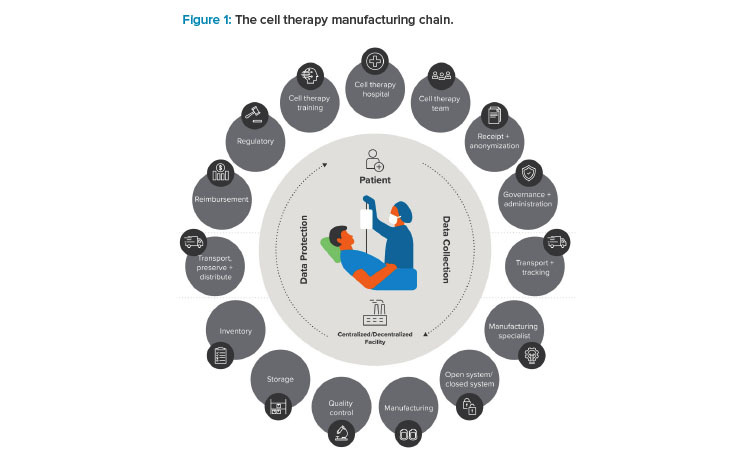
Improved Access Through Shared Knowledge
In the traditional biologics industry, confidentiality is often prioritized over collaboration. Large, well-established companies rely on proprietary intellectual property to compete for global market share. The ATMP industry is different: to move forward against the headwinds of significant manufacturing and distribution challenges, stakeholders in this industry need open dialogue, adaptive co-learning, and consistent knowledge transfer strategies.7
Cross-functional, ATMP-focused partnerships are emerging around the world in response to that need. The European Union (EU)-funded JOIN4ATMP is a recent example. Launched in early 2024, it “brings together all members of the European University Hospital Alliance with the existing EU-funded T2EVOLVE and RESTORE networks, and with active support from industry partners and patient representatives as core partners” with the goal of ensuring “a holistic assessment of current hurdles and the best way to improve support and regulation of ATMP development and use.”8, 9
Consortiums like this are the key to addressing the long timelines, significant complexities, and high costs associated with the ATMP manufacturing chain.10 By working together to understand these challenges, stakeholders across this industry can multiply their positive impact across diverse fronts. The JOIN4ATMP consortium, for example, aims to drive outcomes from the perspective of scientific advancement, quality of life for patients, social and structural improvements, and economic and technological gains..11
Scalability Challenges
For manufacturers of personalized therapies, each batch is patient-specific, precluding the possibility of bulk scaleup. Multi-stakeholder organizations, like the Alliance for Regenerative Medicine, seek to help ATMP manufacturers address scalability issues. This is done by understanding current manufacturing constraints and codeveloping solutions in response..12, 13
Complexity of the Manufacturing Process
With ATMPs still relatively new compared to other life science modalities, many companies in this submarket have not yet fully characterized and standardized their manufacturing processes, leaving them reliant on manual interventions. This has added to the challenge of transitioning from research and development (R&D) to a commercially viable, GMP-ready manufacturing platform.14
In response to these manufacturing challenges, many of today’s ATMP research teams are working in partnership with equipment vendors and point-of-care institutions looking for solutions. Robotically driven ATMP manufacturing platforms are one promising result of these partnerships, making it possible for manufacturers to automate their processes and thereby improve their reliability and scalability..15
Regulatory Hurdles
The streamlined approval pathways designed to accelerate compliance for traditional drug manufacturers don’t always apply in the context of these novel therapies. Regulatory requirements may vary from region to region, for example, and different agencies may update their regulations at different times, creating a lag between ATMP innovations and related regulations.16 Challenges like these can make it difficult for manufacturers to navigate the regulatory environment. For example, the “EU GMP Annex 1: Manufacture of Sterile Medicinal Products” guideline, which was recently updated, may apply to ATMPs, which require aseptic handling. However, the European Commission’s stand-alone ATMP guidance states that no other annexes are applicable.17
Regulators and manufacturers must face these compliance challenges together. By engaging with each other early, these stakeholder groups can codevelop and validate a strong risk-based approach to commercial-scale ATMP production. This approach can help prioritize consistency, quality, and, above all, patient safety. 16
Supply Chain Complexity
Once collected from a patient or donor via apheresis or biopsy, the cellular material that begins a cell therapy manufacturing process is typically cryopreserved and transported to the manufacturing site for processing (or transported “fresh” if it can reach the manufacturing facility rapidly). Once the production process is complete, the sensitive live-cell therapies follow the same journey in reverse, moving from the manufacturing site to the patient’s bedside for administration.
Extensive cold chain logistics are required to preserve the quality of these “living” materials while they’re in transit or storage, including robust temperature and storage controls. Manufacturers must also establish a sophisticated end-to-end tracking system to maintain chain of identity and chain of custody, especially when handling personalized therapies where the consequences of a misidentification or mix-up are severe. By working closely with logistics partners and healthcare institutions, manufacturers can codevelop logistics and supply innovations that aim to simplify these dynamics and accelerate the manufacturing life cycle.
The Future of ATMP Manufacturing
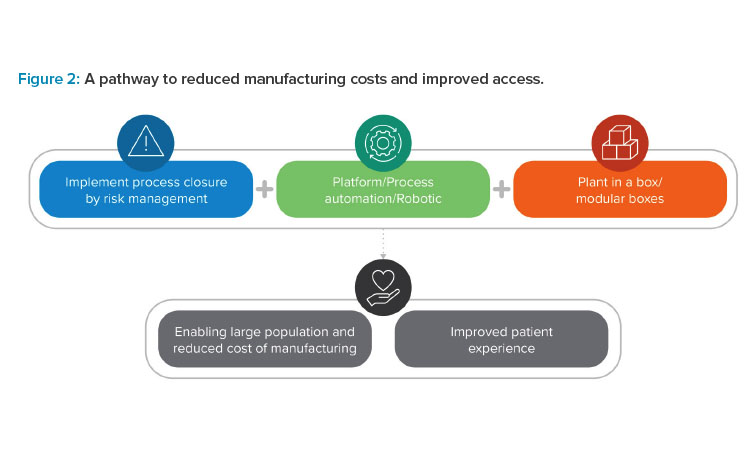
If these challenges seem insurmountable, take heart from reflecting on the biotech boom of the 1980s, when the first monoclonal antibodies (mAbs) emerged on the market. At the time, the process of making these pioneering therapies was lengthy, manual, and highly customized, which elevated their market price.18 After four decades of innovation and improvement, the mAb manufacturing process is well-understood and highly standardized.
Current ATMP pioneers are at the beginning of a similar evolutionary curve. As illustrated in Figure 2, this evolution will depend on technological advances that enable future scalability and speed through closed, automated, and modular processing. Cross-industry collaboration and shared knowledge are the keys to making this future a reality.
To understand how today’s ATMP manufacturers might prepare for this future, the following sections will examine a few of the many vital trends and advancements currently shaping this submarket, particularly in the area of personalized cell therapies.
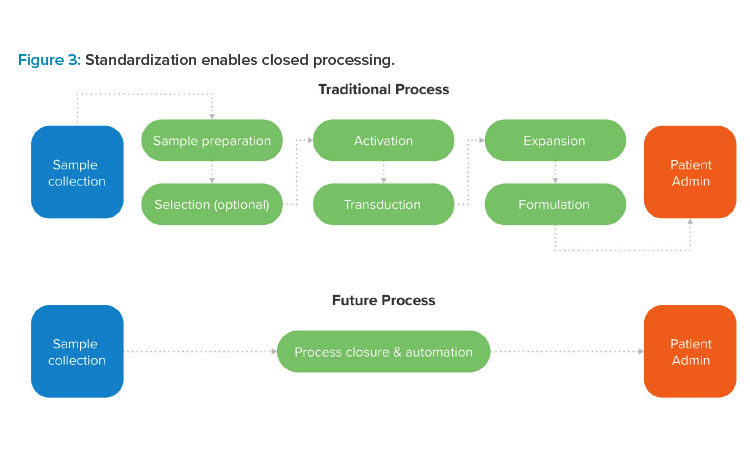
Process Standardization
Here we discuss process standardization for a robotics-ready, automation-optimized cell therapy process. Shifting from customized, operator-driven processes to a standardized process platform is critical to the future of commercial-scale ATMP manufacturing.
Standardization lays the groundwork for a GMP-ready process that yields safe, pure, and reproducible therapies of the highest possible quality.19 Without a standardized process, manual interventions in open processing environments are required. This type of operator-driven approach can drive up the size and cost of production spaces, slow the manufacturing process, and expose in-process substances to the risk of contamination. Standardization addresses these issues by opening the door to automation and robotic or cobotic processing, which is the key to manufacturing safe, pure, and reproducible cell therapies in a scalable GMP environment.
Getting there requires cell therapy innovators to scrutinize their decisions during R&D, looking for ways to eliminate manual actions and develop repeatable protocols that prioritize simplicity, process control, and commercial-scale efficiency.
Closed Process-In-A-Box Systems
Robotics-driven process-in-a-box platforms used within closed processing offer a more scalable and flexible cell therapy manufacturing model. Standardization is the gateway to a manufacturing model that’s transforming the landscape of ATMP manufacturing: process closure.
Historically, manufacturers have mitigated contamination risks by locating open process steps in highly controlled cleanroom environments. But cleanrooms are imperfect—they require energy intensive heating, ventilation, and air conditioning systems; strict operational protocols; and a level of customization that can make them challenging to repurpose as demand shifts.
Closed processing offers an alternative. By leveraging modular process-in-a-box systems, manufacturers can shift the burden of segregation from room-level environmental controls down to the equipment itself. The benefits of such a shift are numerous, particularly for cell therapy manufacturers intent on solving persistent scalability challenges. Closed process-in-a-box systems enable new, more flexible manufacturing models, such as decentralized manufacturing (examined next) and multimodal processing.
By reducing or eliminating the need for strictly controlled environments, process-in-a-box systems also enable simpler and more flexible facilities that cost less to build, run, and maintain. Most importantly, process closure is key to product quality. Through a scientifically driven risk-managed approach, manufacturers can identify the unique risks embedded in their process—risks that may be addressable in part through robust process closure. Because closed systems move process steps into a highly repeatable and automation-driven system of advanced robotic or cobotic technologies, there’s a lower risk of batch-to-batch variability or other quality issues.20
How close is the future of fully automated, robotics-driven process closure for ATMP manufacturers? Several established systems exist today and are often used in the cell therapy context, including Milteny Biotec’s CliniMACS Prodigy,21 Lonza’s Cocoon Platform,22 and Cytiva’s Sefia Select system.23 Other promising systems are currently in development, such as the Constellation by Cellular Origins 23 and a collaboratively developed robotic manufacturing platform by Cellular Origins and Cytiva.24
However, many manufacturers are relying for now on a hybrid approach, in which they leverage closed processing units for automated steps and isolators to accommodate open manipulations. Some manufacturers of specific modalities, such as adeno-associated virus vectors, are even investigating the possibility of using closed production units to enable continuous processing. Experts speculate this advanced strategy may become standard in the future.25
Pharma 4.0™ Driven by Robotics and Other Automation Technologies
Robotics and other automation technologies can be leveraged to enable a more intelligent and efficient operation. Process-in-a-box systems that leverage advanced robotic and cobotic technologies to close the ATMP manufacturing process exemplify an overall industry shift toward Pharma 4.0™—that is, an approach to manufacturing that centers on an ecosystem of data-driven, automated, and scalable systems.
For example, process-in-a-box modules can leverage process analytical technology with a feedback loop. A system of advanced sensors enables this approach, allowing the platform to monitor and analyze critical process parameters and critical quality attributes while processing is underway. Robotic mechanisms inside the platform then use this data to make proactive adjustments, often without manual intervention. Capabilities like this help improve batch consistency, maximize uptime, and facilitate transparent and proactive communications with regulators.
Outside of process-in-a-box implementations, the principles of Pharma 4.0™ may lead to improved patient access. By integrating robotics and artificial intelligence, for example, manufacturers can analyze their operational data to optimize their processes and predict potential maintenance needs or material shortages. This foreknowledge can help manufacturers prevent delivery delays or other issues that drive up the cost of final products. These integrations are also key to reliable traceability, a critical factor for cell therapy manufacturers with extensive and complex supply chains and patient-specific workflows.25
![Figure 4: The pathway to decentralized cell therapy manufacturing [28].](/sites/default/files/2025-05/0525_PE_MJ_Martins_05.jpg)
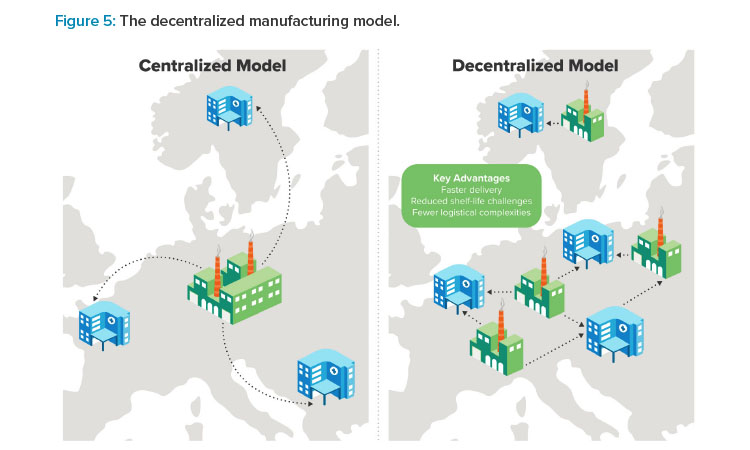
Decentralized Manufacturing
Decentralized manufacturing can be used to support shorter vein-to-vein timelines and stronger cross-functional partnerships. The strategies described earlier invite ATMP manufacturers to consider their manufacturing models from new perspectives and ask questions that could change how cell and gene therapies are delivered to patients. For example, what if ATMP manufacturers leveraged standardized, robotically driven, user-friendly process-in-a-box systems to move production as close as possible to the patient’s bedside?
This vision is driving manufacturers toward decentralized manufacturing for certain cell and gene therapy modalities.25 The idea is to leverage the mobile and compact nature of closed processing units to untether the manufacturing process from large, centrally located facilities, putting them instead right inside (or adjacent to) hospitals, specialized treatment centers, and other points of care.
Evidence suggests that a distributed manufacturing network supports good patient outcomes by potentially cutting vein-to-vein timelines in half.26 Regulatory agencies are recognizing the benefit of embracing decentralized manufacturing in some cases; under the Hospital Exemption Clause, for example, the European Medicines Agency authorized local Spanish academic institutions to manufacture CAR T cell therapies outside of the EU’s standard centralized marketing authorization pathway.27
Manufacturers know what they need to get there. When asked in a 2023 industry survey, 500 respondents identified solutions and strategies such as deeper process automation, early regulatory engagement, and alignment with hospital groups as necessary enablers of a decentralized model.28 Each of these changes will rely on a commitment to knowledge sharing and co-discovery across the industry.
Just as shared knowledge is an enabler of decentralized ATMP manufacturing, so too is it an outcome. Removing the literal walls between traditional manufacturing sites and patient care sites means that ATMP labs, R&D teams, manufacturers, academic institutions, and hospitals will benefit from less fragmentation and more collaborative problem-solving. At the Medical College of Wisconsin, for example, the Cell Therapy Shared Resource allows researchers and clinicians to work closely together without needing their own GMP manufacturing facilities. Instead, they leverage shared, decentralized cell therapy processing and production infrastructure to manufacture CAR T cell therapies on-site.29
Applications for this decentralized approach are growing as closed processing technologies continue to evolve. Biotech companies such as Orgenesis are bringing decentralized cell processing to market, with platforms intended to transform the future of ATMP manufacturing.30 Elsewhere, the collaborative network BioCanRx is currently expanding its decentralized CAR T cell manufacturing capabilities across Canada.31 Examples like these abound, demonstrating the momentum behind decentralized manufacturing and its potential to shorten timelines, lower manufacturing costs, and improve patient access.
To fully embrace a decentralized model, manufacturers must first address several challenges, including issues related to quality control and concerns about the protection of intellectual property. From a quality perspective, manufacturers have several factors to consider, with solutions available given adequate and early planning.
Regulatory compliance is a chief concern. Ensuring compliance across multiple localized sites requires robust standardized processes supported by a consistent quality management system (QMS). This system should be monitored and enforced via regular audits and compliance checks.32 Establishing a contamination control strategy (CCS) within the framework of that QMS is key. A strong CCS helps manufacturers identify and mitigate high bioburden levels and other quality-related risks, thereby protecting patient safety and ensuring ongoing regulatory compliance.33
Decentralized manufacturing must also address the potential for variability in local skill sets. The closed and automated technologies described in Section 2 are the key to addressing these risks by minimizing or eliminating the need for human intervention.34 Supply chain complexity also grows alongside decentralized manufacturing networks. To manage the risk of a supply-related delay, manufacturers need to establish strategic cold chain logistics supported by real-time tracking systems. Finally, integrating data from many localized manufacturing sites into a single main system to monitor for quality and consistency may be a challenge for decentralized manufacturers. Secure cloud-based platforms designed for real-time data exchange are a vital solution..35
Maintaining integrity around proprietary information is also a chief concern. This is especially true as manufacturers shift from a centrally controlled manufacturing center to a distributed model. In these models, point-of- care institutions are entrusted with some or all elements of the manufacturing process. 36
A strong licensing strategy is one part of the solution. By licensing the production process, manufacturers can ensure that point-of-care institutions, such as teaching hospitals, have the knowledge they need to manufacture ATMPs while proprietary information remains protected. Regular audits are necessary to ensure ongoing compliance with licensing terms..37
Embedding trained personnel in decentralized manufacturing sites is another useful strategy. These experts can oversee the production process and ensure adherence to protocols (including those designed to protect proprietary information). Using embedded personnel in combination with a system for continuous remote monitoring may help to decrease the risk of security-related compliance issues.
Technology-driven protection measures also have an important role to play in protecting proprietary information within a decentralized manufacturing model. Manufacturers need to implement robust data encryption systems to protect digital information, as well as strict access controls to prevent information leakage. 38
Operational Improvement
Operational improvement (OI) is key in existing or new facilities for streamlined and scalable workflows. A well-designed OI strategy can help ATMP manufacturers understand, troubleshoot, and optimize the unique systems and processes involved in cell and gene therapy production.39 For manufacturers planning a new commercial-scale ATMP facility, investing in OI during the early design phase will ensure that every capital dollar spent today generates value long into the future. Manufacturers with established facilities can use their OI strategy to proactively identify and eliminate issues before they happen and maximize the performance of their existing systems.
Robust OI studies rely on several advanced tools, each contributing to long-term process optimization. The digital twin is a virtual duplication or computational model of a specific manufacturing process or the facility as a whole. Using that digital twin, ATMP manufacturers can leverage modeling and simulation to troubleshoot performance issues (in an existing facility) or test scenarios before committing to a facility layout or investing in new equipment.
Process monitoring models can direct manufacturers toward emerging trends and improvements that will reduce out-of-specification events and avoid conformance issues. Discrete event simulations go even further, allowing manufacturers to incorporate uncertainty into their simulations. This is an especially useful tool for ATMP manufacturers, who face uncertainty on multiple levels, from sudden shifts in demand that make capacity alignment difficult to transport issues that delay the start of new batches.
Manufacturers can use these OI tools to continuously evaluate and address issues that impact the duration, complexity, and reliability of the ATMP production process. For example, simulations can help establish an equipment utilization strategy that balances efficiency with the need for risk-based redundancy planning to avoid bottlenecks or unplanned downtime. These tools are also vital in scheduling maintenance and cleaning activities, optimizing staffing strategies, streamlining the flow of personnel through the facility, increasing warehouse and storage efficiencies, optimizing quality control testing protocols, and much more.
Conclusion
Knowledge sharing is the key to accelerating ATMP research, broadening patient access, and reducing costs. The ATMP industry can overcome the challenges it faces by removing silos within the scientific community and inviting a meaningful exchange of knowledge and insight between academic research bodies, manufacturers, equipment suppliers, regulatory agencies, and public healthcare institutions. Already, examples of research breakthroughs accelerated by collaboration abound.
Developers of cutting-edge manufacturing technologies are working closely with cell and gene therapy research and development accelerators, each intent on improving patient access through novel strategies and solutions, such as process-in-a-box technologies.40, 41 Collaborations like these are the key to unlocking the full promise of ATMPs. Only by sharing ideas and working together to develop simpler, more flexible, and less costly manufacturing pathways can ATMP manufacturers ensure access to high-quality cell and gene therapies for every patient in need.



