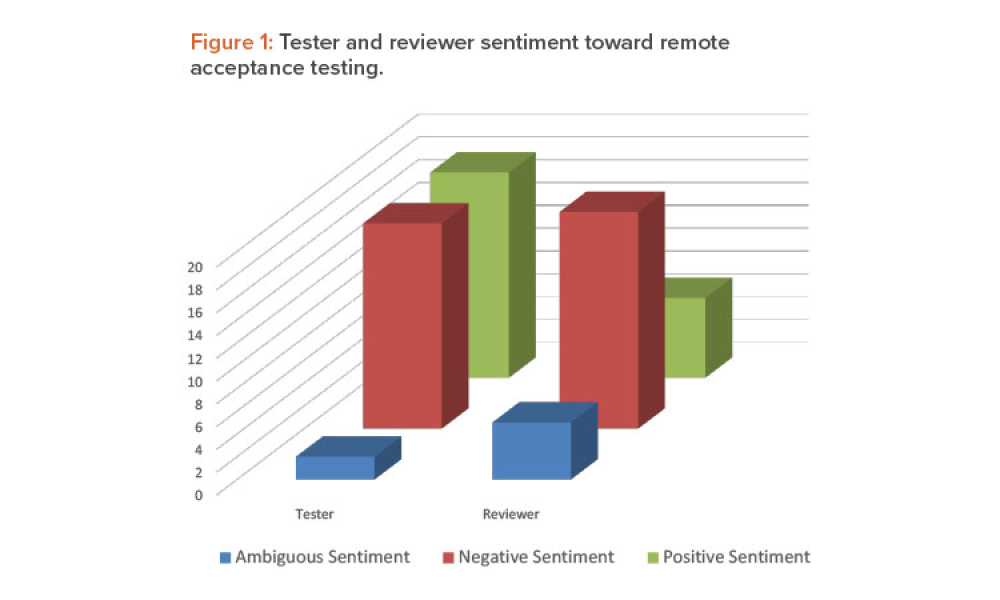
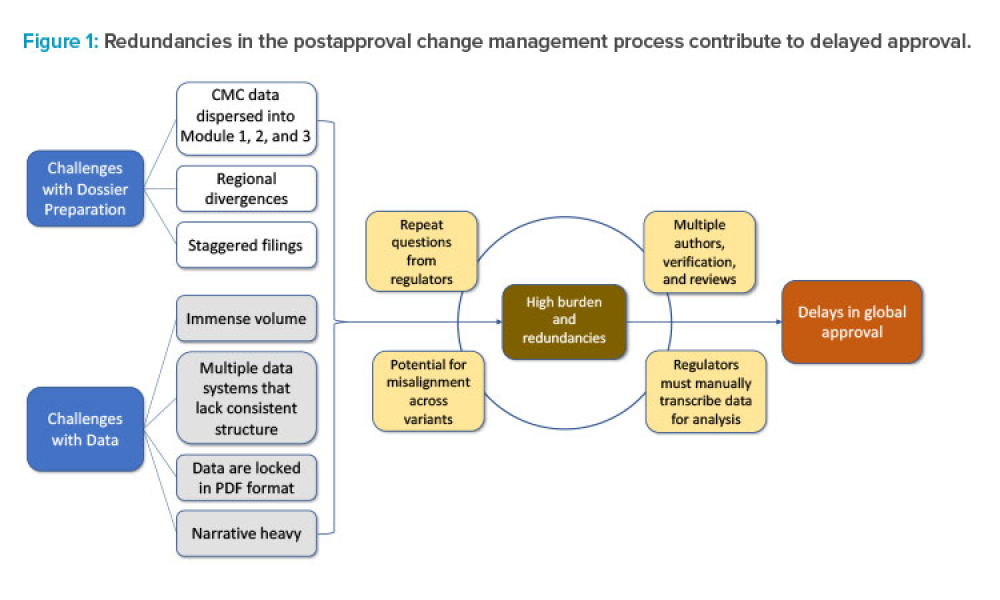
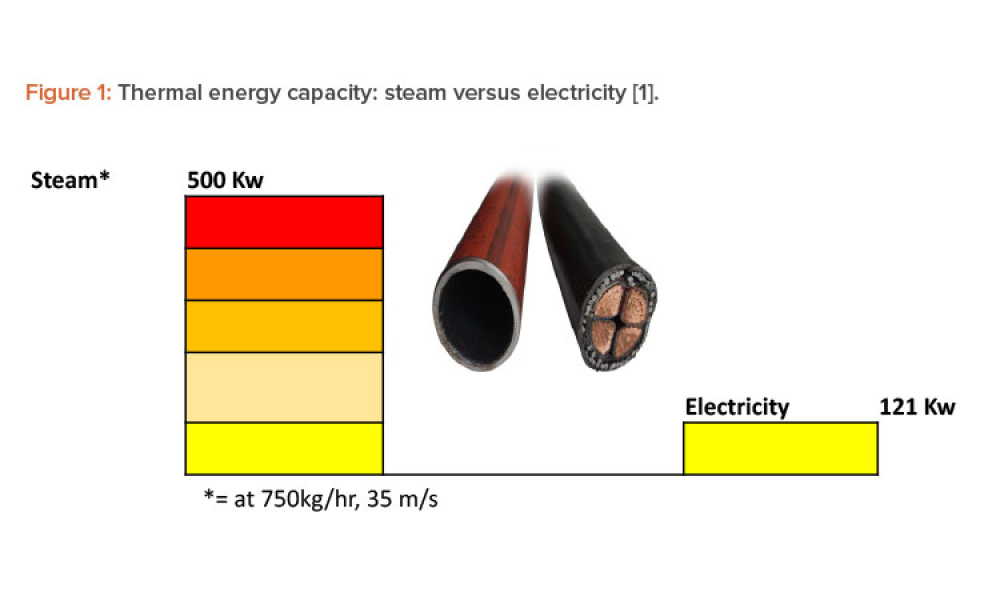
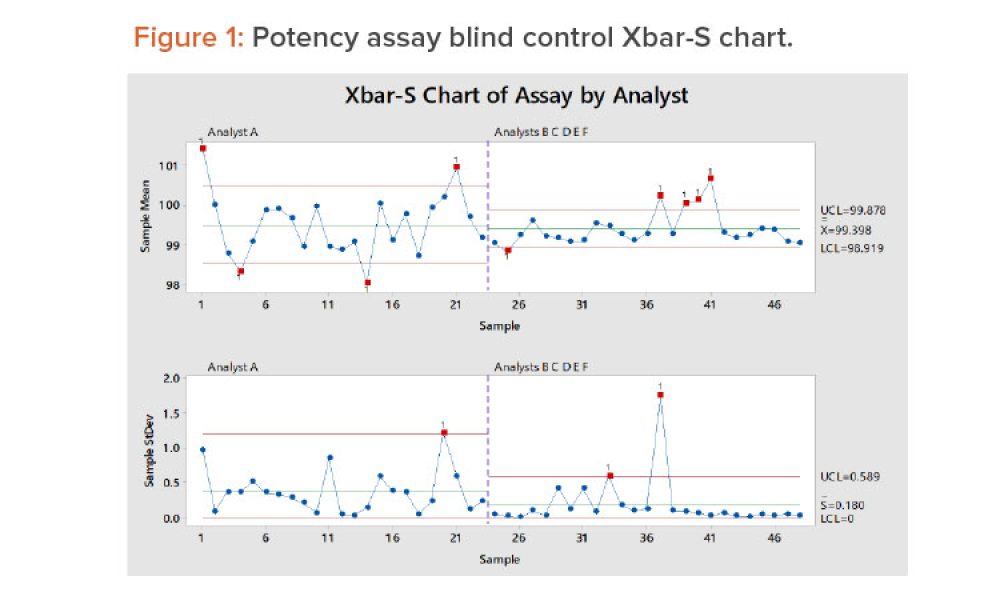
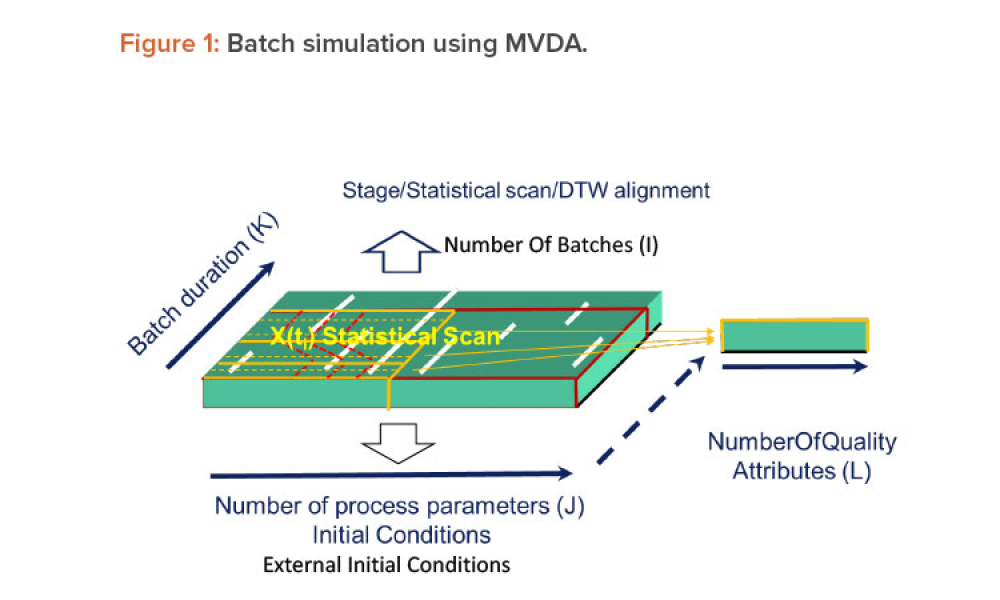
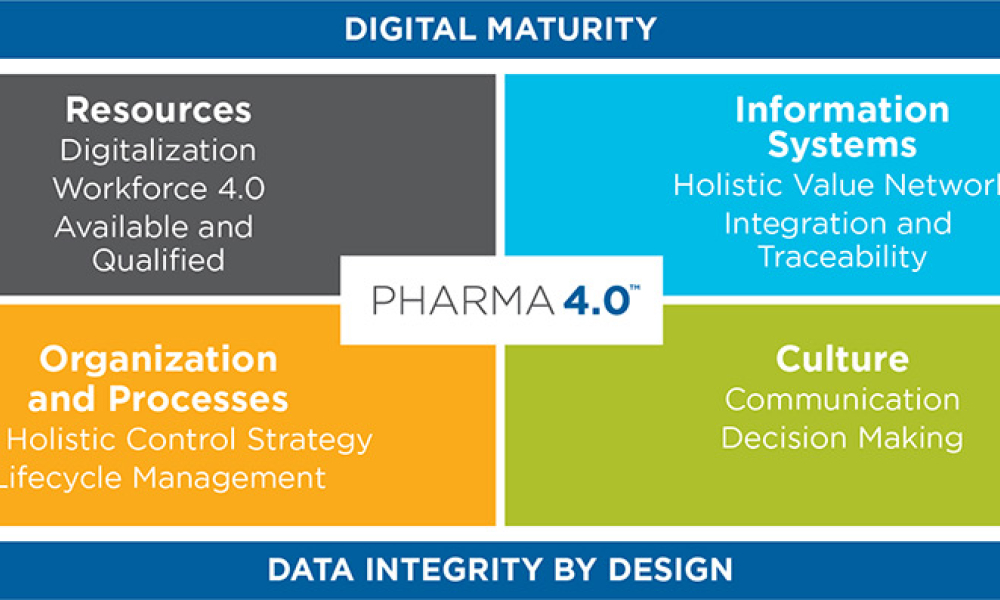
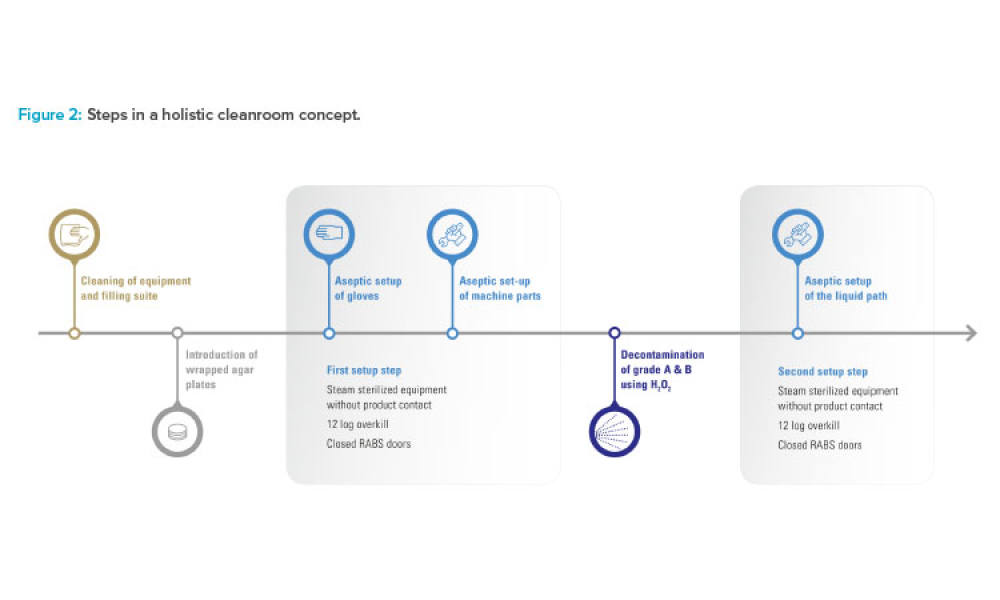
The ISPE Commissioning & Qualification (C&Q) Community of Practice (CoP) conducted a survey in 2023 on the adoption of integrated C&Q, specifically on the use of paperless digital systems for planning, executing, and reporting C&Q activities. The survey revealed that 74% of respondents planned to use Digital Validation Tools (DVTs) for C&Q by 2024.
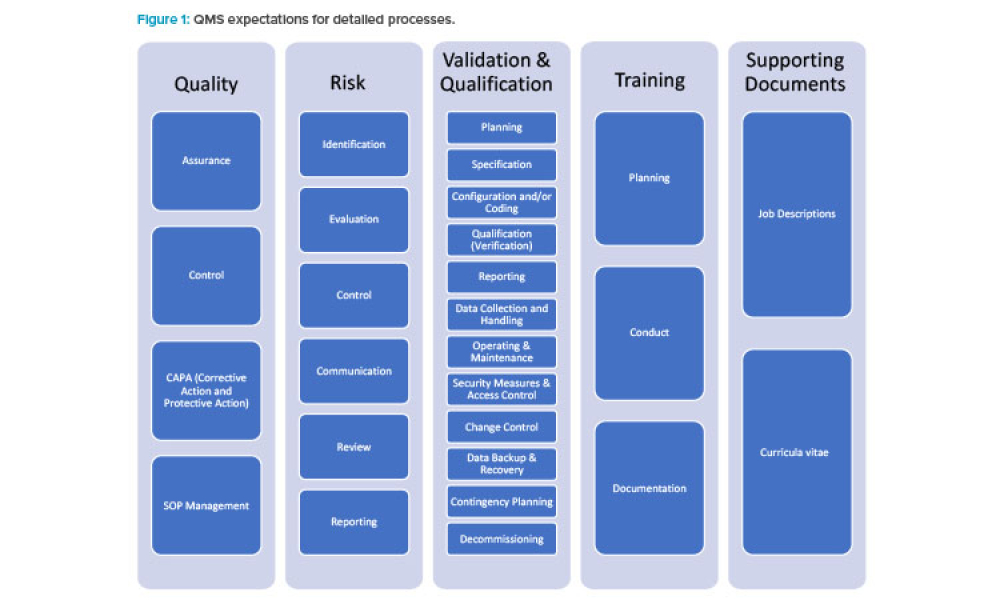
Integrating Environmental Monitoring System (EMS) data into your Quality Management System (QMS) isn't just about compliance—it’s about building a robust foundation for risk management, process optimization, and product integrity.