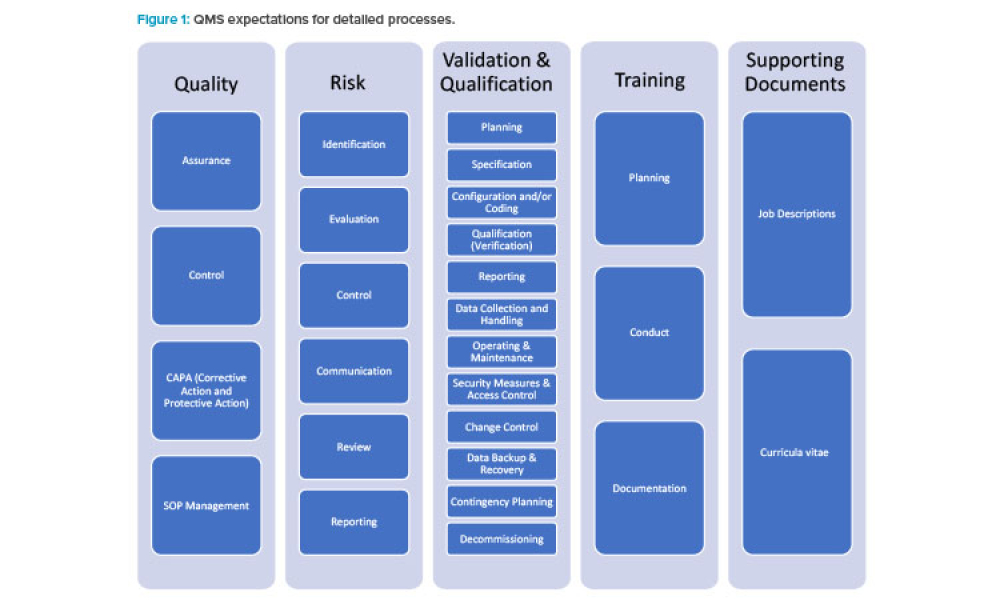
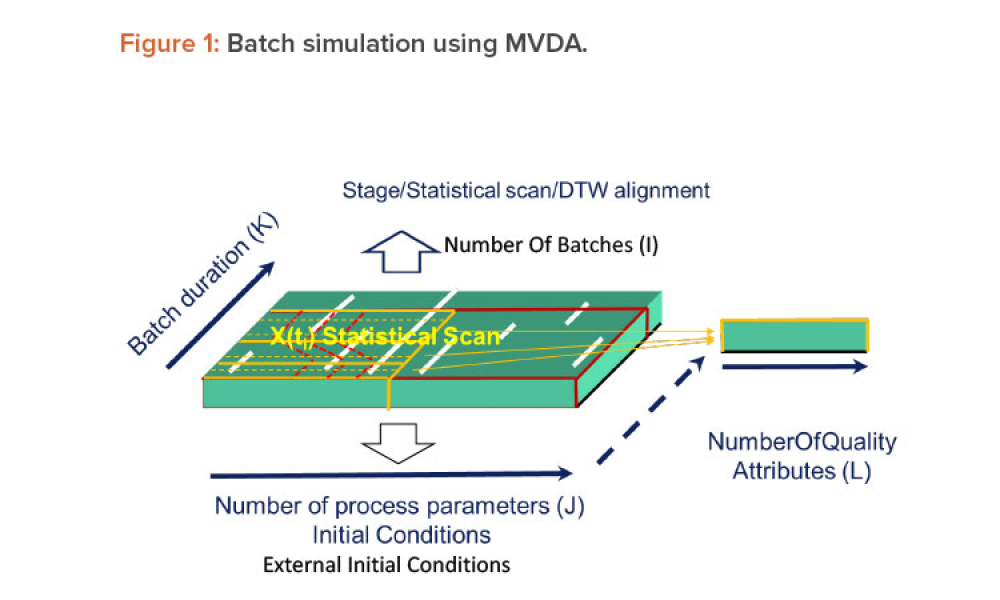
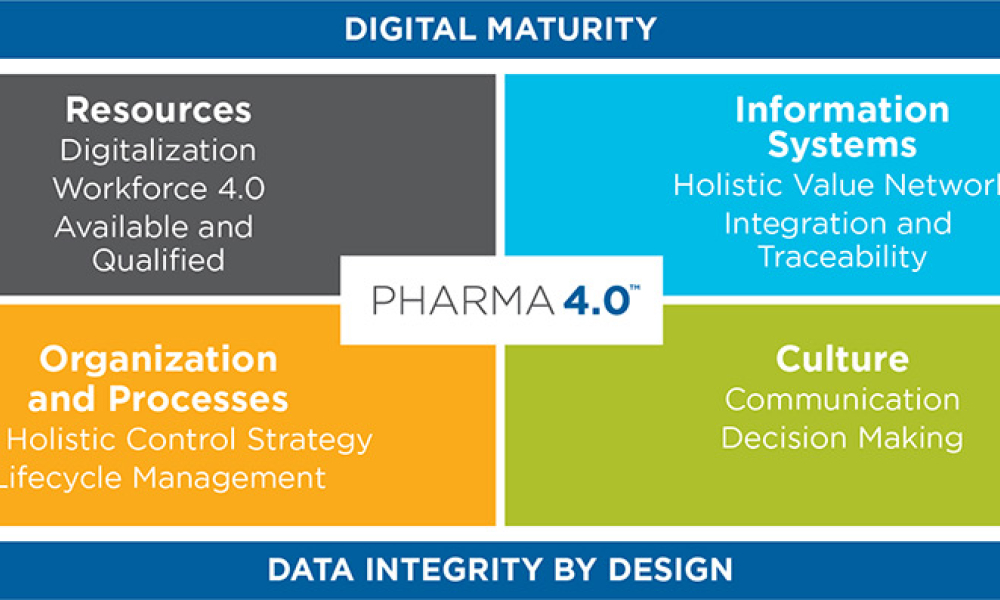
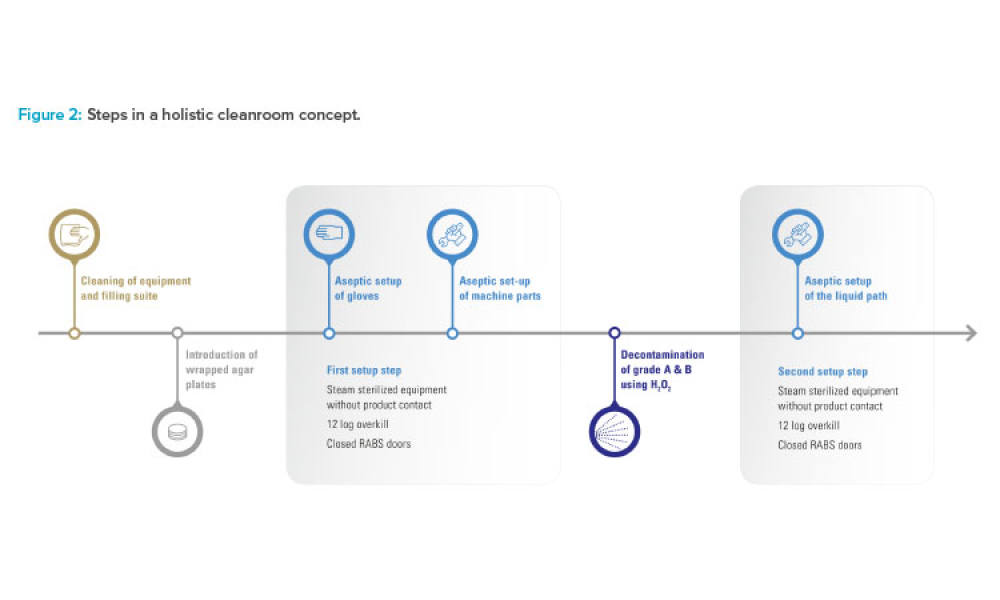
As the pharma industry moves to an ambitious Validation 4.0 paradigm, computerized systems play a pivotal role in enabling the rapid transition. Innovation and agility in computerized system validation (CSV) received a strong push in the second half of 2022 with the publication of the FDA draft guidance on “Computer Software Assurance for Production and Quality System Software”
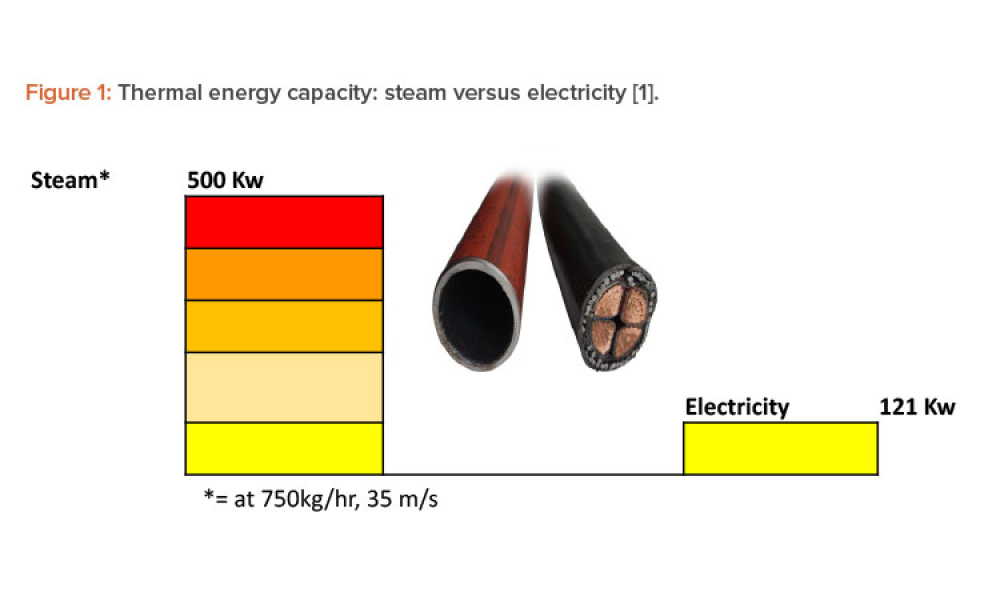
The world is beginning to grasp the huge challenge of achieving net-zero carbon emissions, or carbon neutrality, by 2050. Many countries have committed to achieving this ambitious goal. As a major global industry, the pharmaceutical sector has a significant role to play. For thermal energy–intensive industries, such as pharmaceutical manufacturing, the long-term future options to maintain...