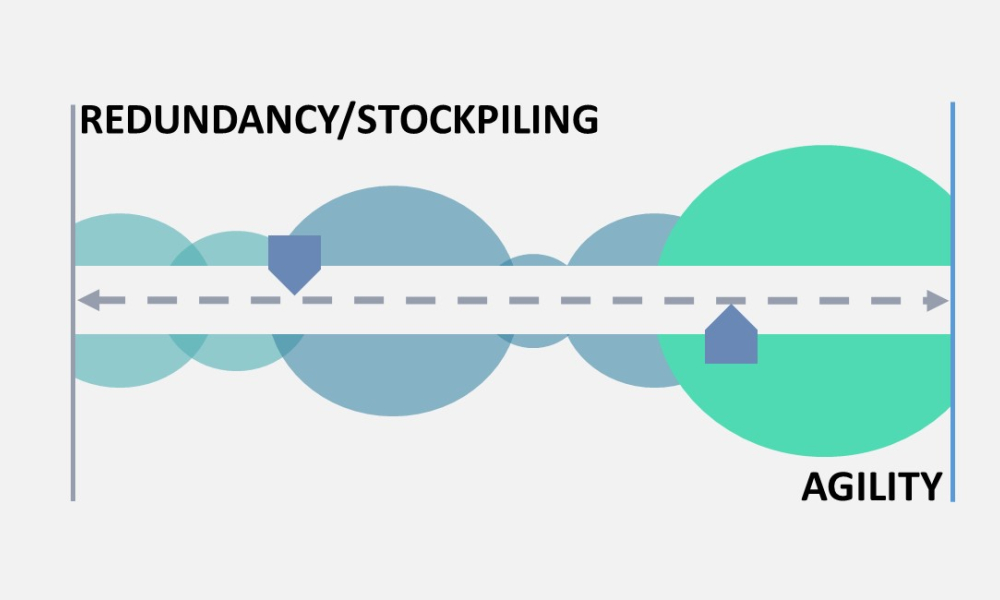
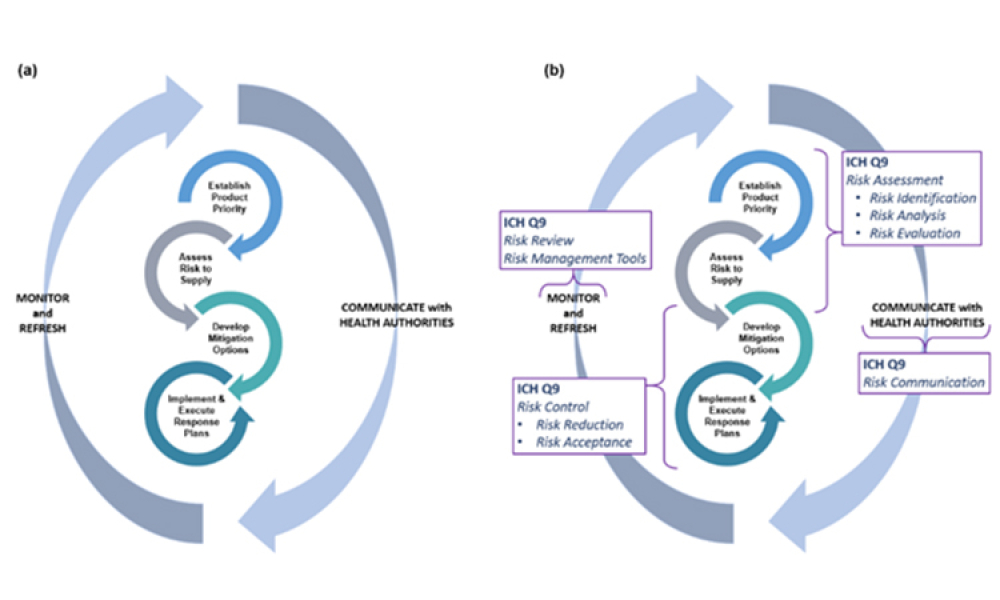
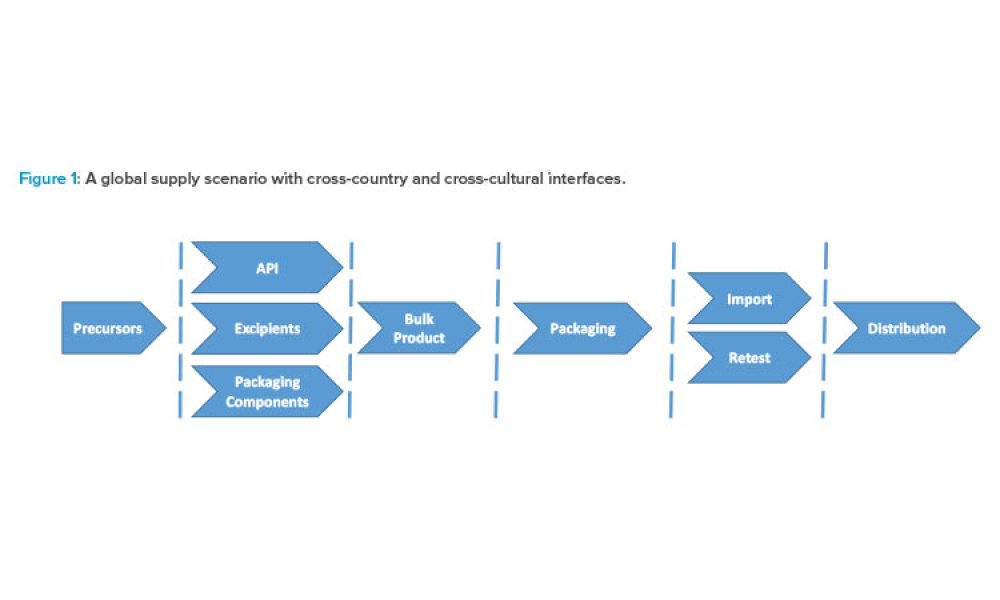
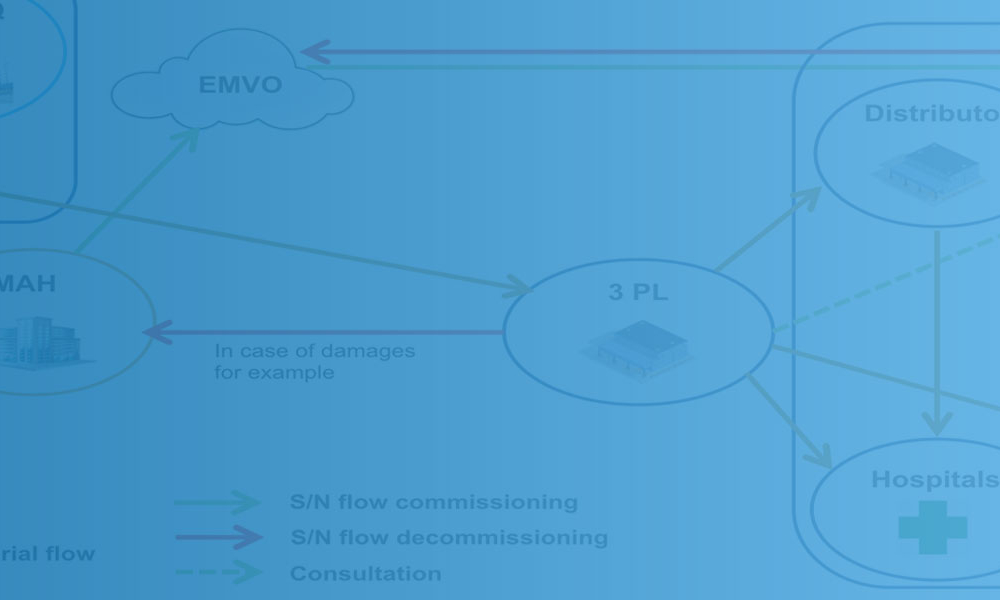
Advanced therapy medicinal products (ATMPs) are one of the most promising developments in the pharmaceutical and biotech industries in recent decades. Although there is a great promise to treat and even cure many diseases with these products, there are also unique challenges, especially with their supply chains.
The global supply network that underpins life science manufacturing is vast and highly complex. Maintaining a reliable inflow of critical materials depends on more than your relationship with a particular supplier—it also depends on that supplier’s supply chain, with further dependencies and variabilities all along the value chain. With contract manufacturers also in the mix, the complexity...