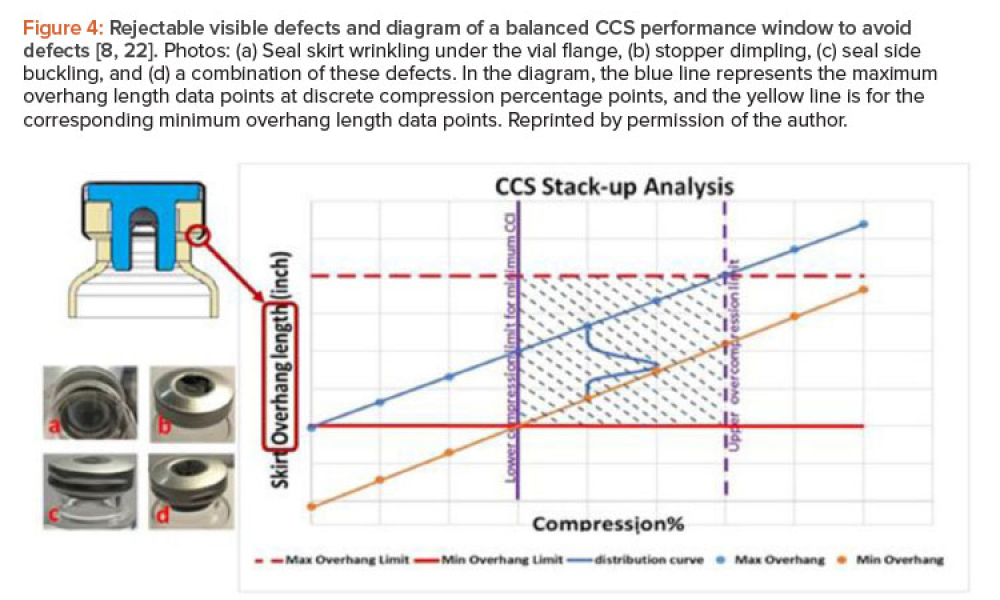
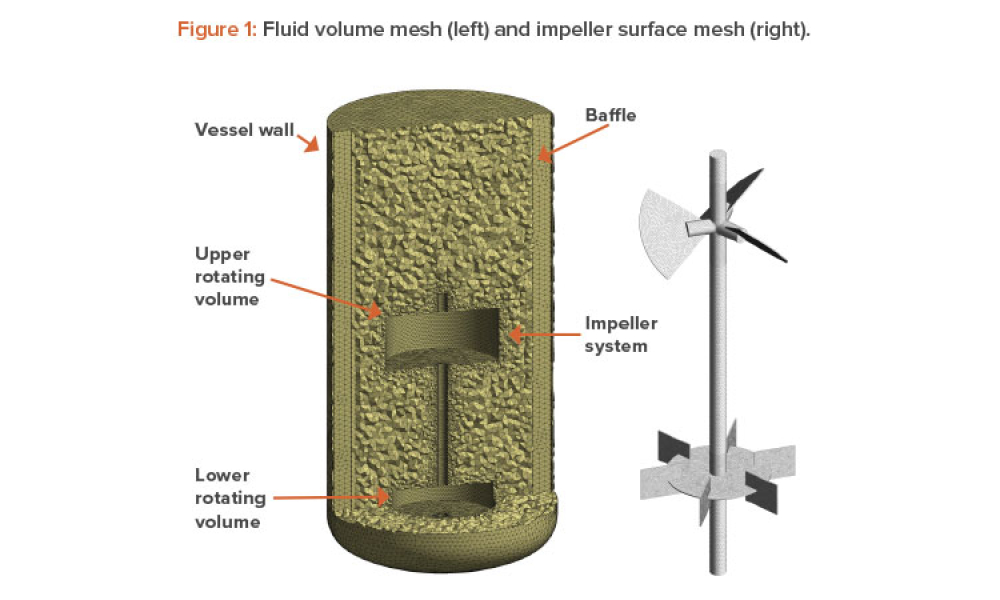
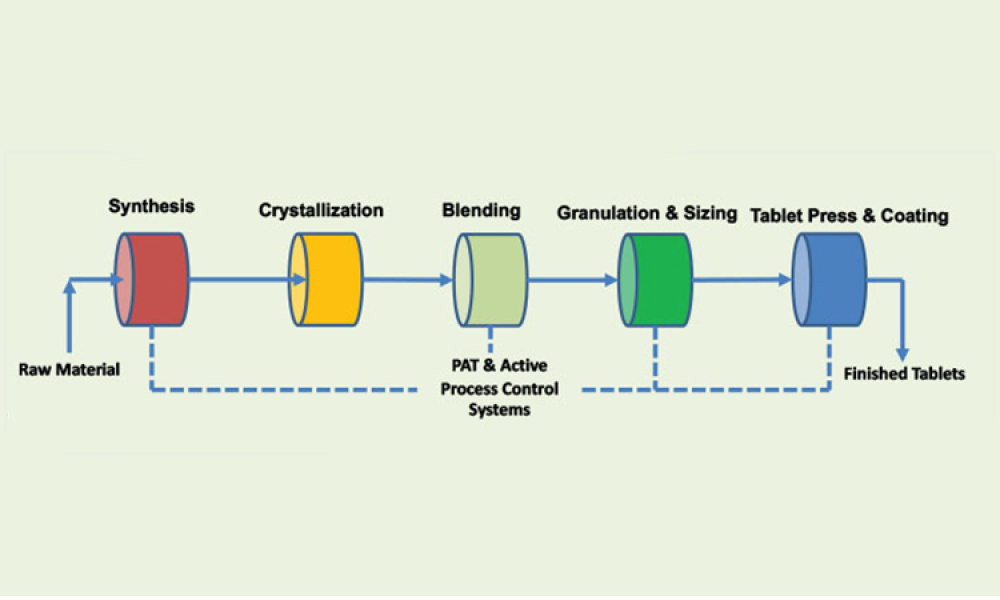
Any systematic pharmaceutical engineering approach for ensuring vial container closure system (CCS) performance must include choosing qualified container closure system components, the proper pharmaceutical process setup, and applicable testing methods. Container closure integrity (CCI) is an essential part of container closure system performance. A holistic strategy is needed to qualify...
Robotic process automation (RPA) software automatically handles manual, repetitive, time-consuming, and highly structured tasks such as data entry and back-office functions. Certain processes specific to the pharmaceutical industry represent strong candidates for RPA implementation, with significant potential savings and the possibility of ensuring compliance.