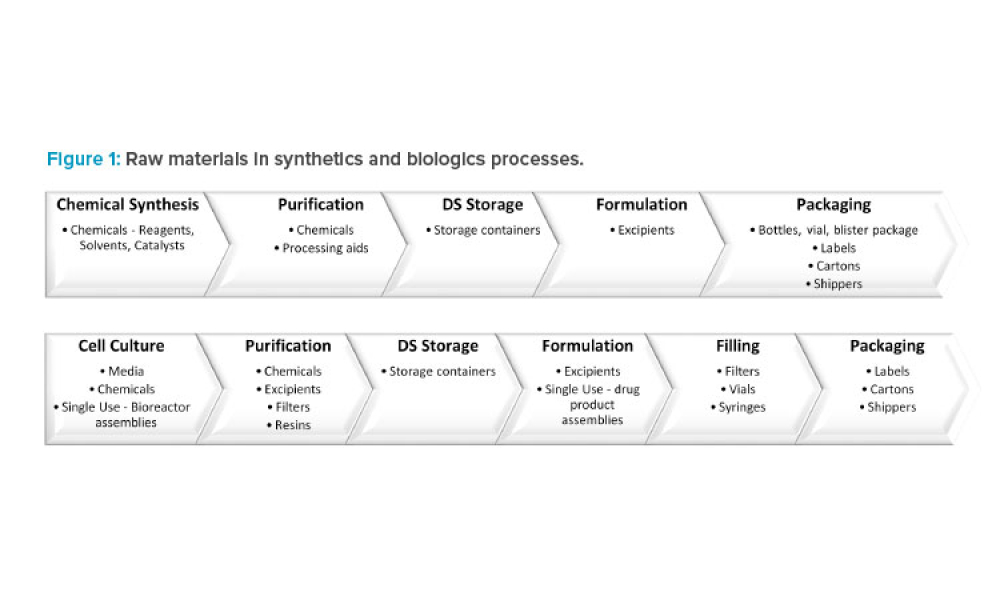
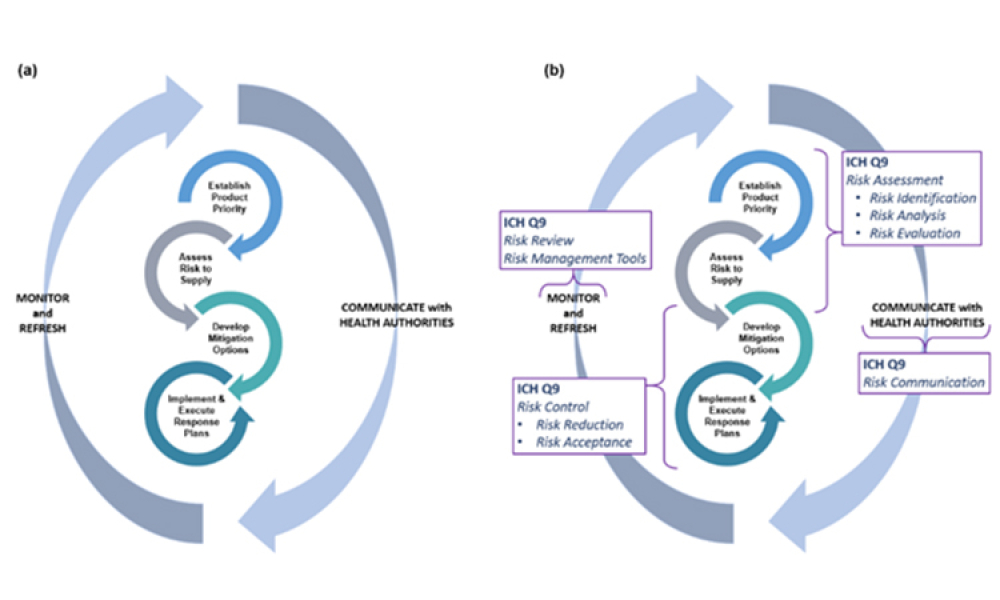
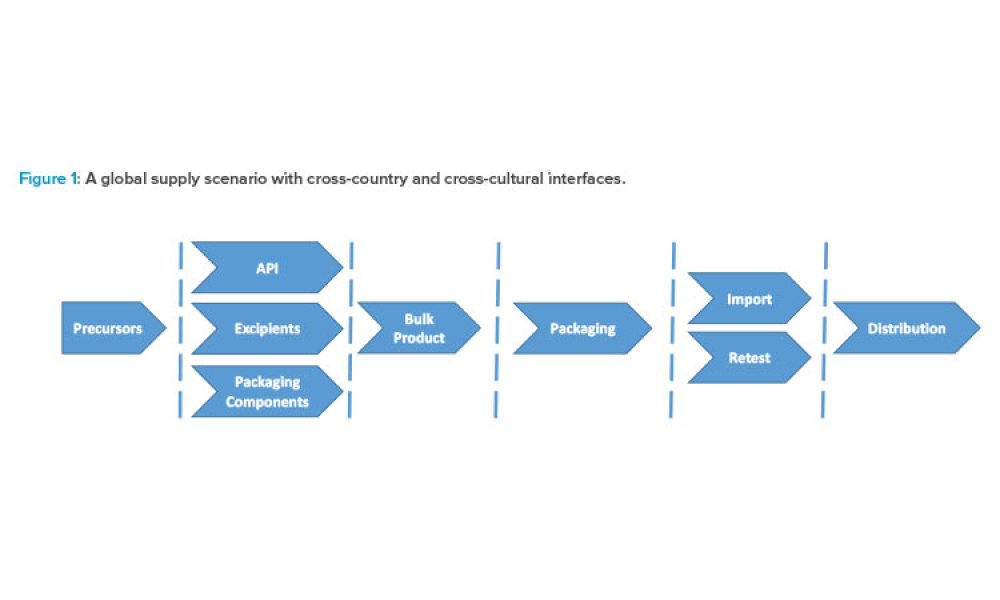
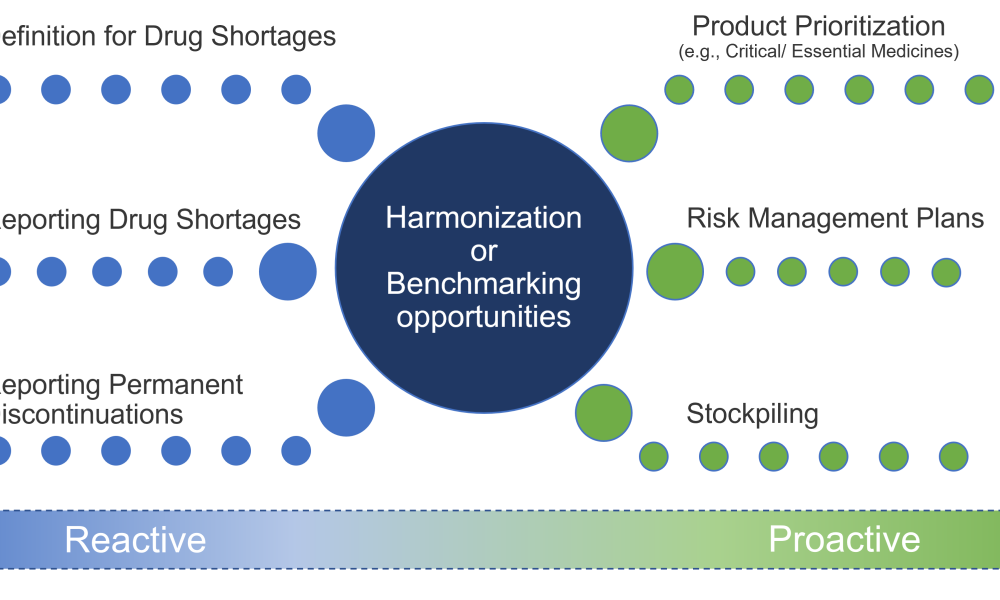
Content focuses on feature key components and the financial impact of supply and distribution chains. Topics focus on the systems required to control and automate the receipt, storage, and dispensation of raw and packaging materials and the storage and distribution of finished products. Other topics could include materials management, operational economics, and warehouse and distribution management.
Guidance Documents
Active Pharmaceutical Ingredients (1)
+Drug Shortages (1)
+Investigational Products (1)
+Packaging (1)
+Project Management (1)
+Regulatory (1)
+Single Use Technologies & Disposables (1)
+Supply Chain Management (6)
+Sustainable Facilities, HVAC, & Controlled Environments (1)
+Community Discussions
Community Discussions
May 22, 2025
Apr 24, 2025
Validation
Apr 16, 2025
Information Systems
Regulatory
Advanced Manufacturing
Artificial Intelligence
Apr 08, 2025
Data Integrity
Apr 07, 2025
Advanced Manufacturing
Artificial Intelligence
iSpeak Blog Posts
Webinars
Upcoming
On-Demand
ISPE in the News
Latest
-
Navigating the Horizon: ISPE’s Vision for Tomorrow
-
ISPE Academy: GMP Refresher Certificate Program
-
Startup Raises $5.5M for Precision Glycans
-
EMA Proposes Updates to Guidance for Cell-, Gene-, Tissue-Based Therapies
-
US FDA Issues Draft Guidance for Color Additives in Drugs
-
Dawah Pharma Partners with Egypt for Supplements, Pharma Products Production
-
Made Scientific Expands Cell Therapy Manufacturing Space
-
Fujifilm's Tilburg Site Goes Greener with e-Boiler
-
Automating Bioprocesses Without Sacrificing Flexibility
- ISPE GAMP® Good Practice Guide: Validation and Compliance of Computerized GCP Systems and Data - Good eClinical Practice
Pharmaceutical Engineering Magazine Articles
White Papers
March / April 2023
As the pharmaceutical industry faces ever-changing global challenges and market forces, it must…

Videos
Professional Development Training
GMP Fundamentals: Holding & Distribution
+Featured Conferences