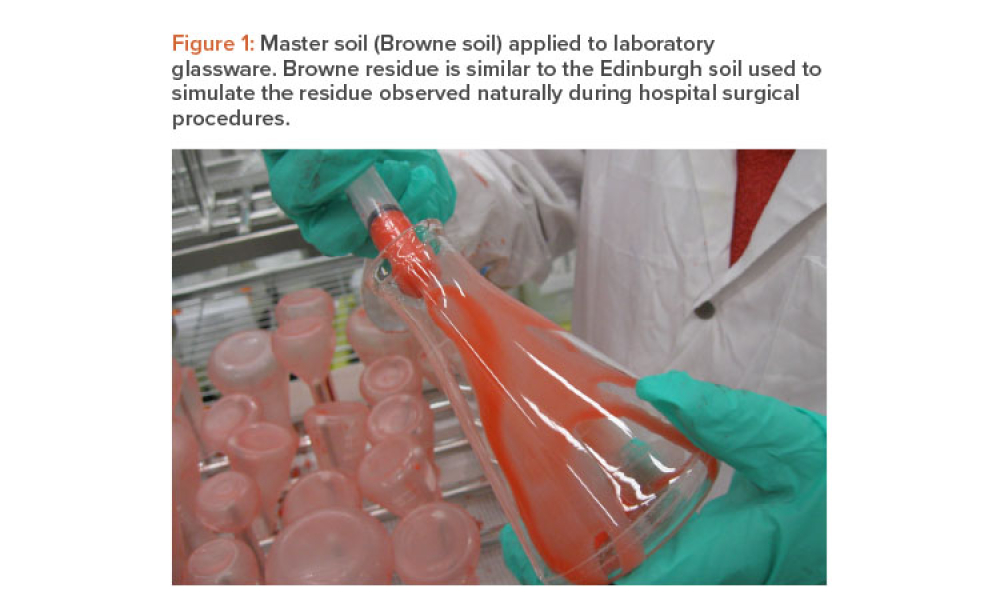
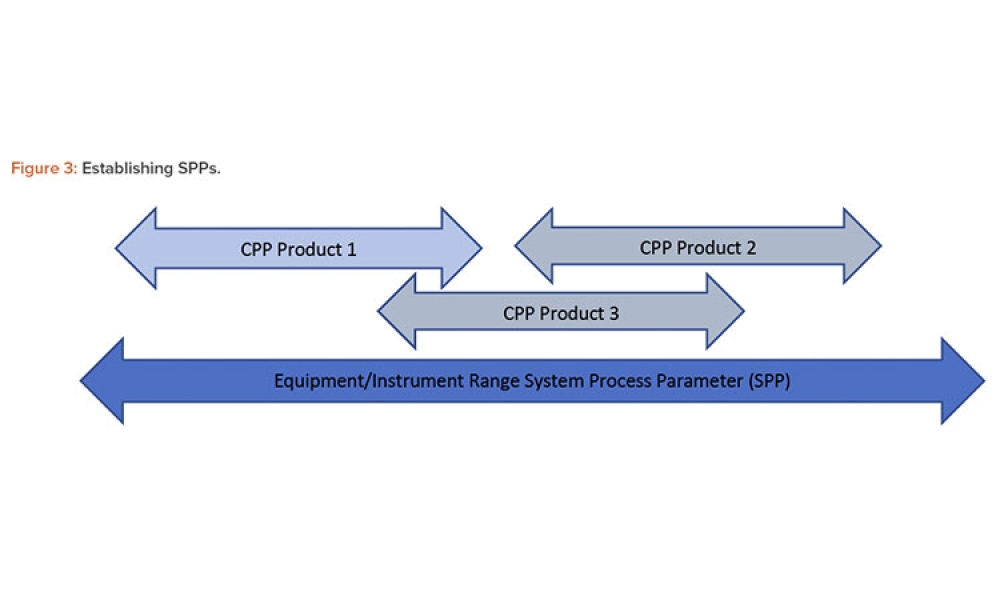
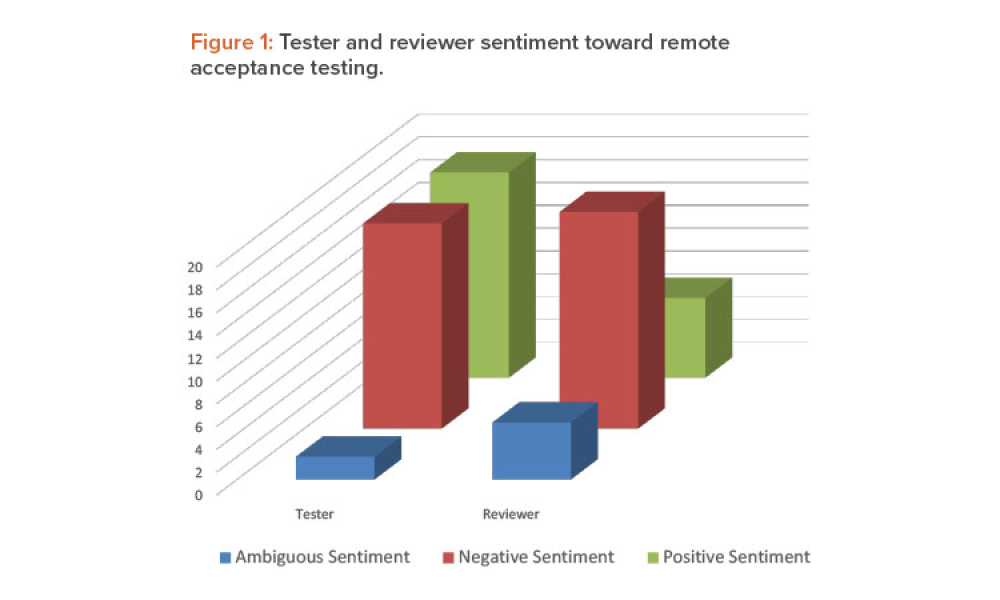
Commissioning and Qualification (C&Q) are terms and processes related to the manufacturing of pharmaceutical or biotechnology products. Each term represents a scope of work that is part of a larger framework for making sure that a facility —and the equipment in it— will function as required and be approved by the regulatory agencies that have jurisdiction over that facility. Produced by pharmaceutical manufacturing industry professionals, ISPE offers a variety of resources to help narrow interpretation of regulatory standards for improved compliance and quality, efficiency, and cost reductions.
Join the Conversation
As an ISPE member you can engage with 22 active CoPs, including new communities focused on Artificial Intelligence and Sustainability. Connect with experts and join the conversations: Become an ISPE member
ISPE members: Get more involved by volunteering.
Guidance Documents
Commissioning & Qualification (6)
+Critical Utilities (1)
+Data Integrity (1)
+GAMP® (1)
+Quality by Design (1)
+Regulatory (2)
+Sustainable Facilities, HVAC, & Controlled Environments (2)
+Community Discussions
Community Discussions
Webinars
iSpeak Blog Posts
Pharmaceutical Engineering Magazine Articles
Videos
Professional Development Training
Good Engineering Practice (GEP) Training Course
+Commissioning and Qualification Training Course
+Facilities Management Training Course
+Pharma Facilities Project Management Training Course
+Pharmaceutical Job Board
Featured Conferences