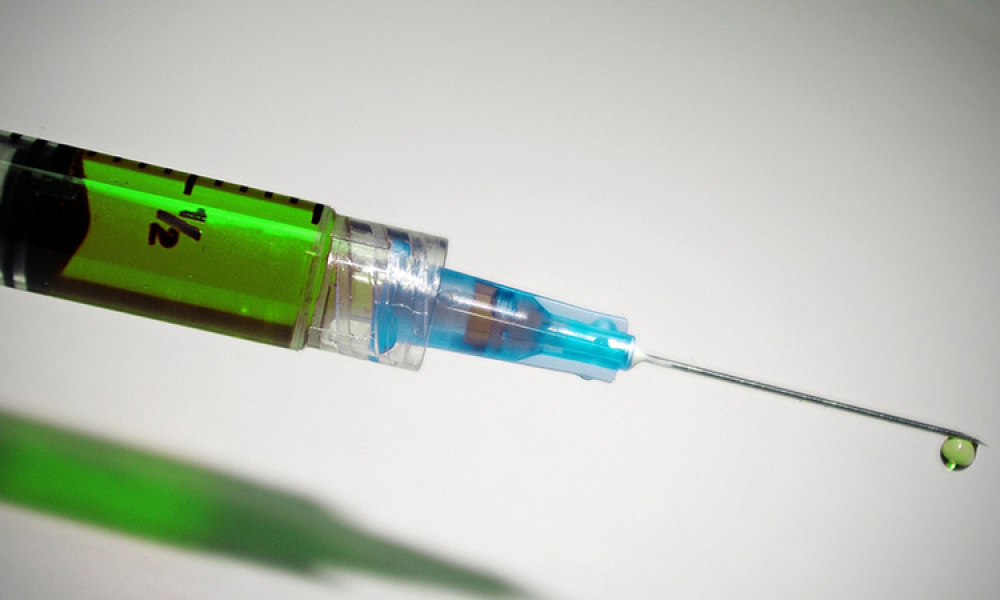
A Combination Product (Combo Prod) is a product composed of any combination of a drug and a device; a biological product and a device; a drug and a biological product; or a drug, device, and a biological product.
Volunteer Callout
Join the Conversation
As an ISPE member you can engage with 22 active CoPs, including new communities focused on Artificial Intelligence and Sustainability. Connect with experts and join the conversations: Become an ISPE member
ISPE members: Get more involved by volunteering.
Guidance Documents
Active Pharmaceutical Ingredients (3)
+Advanced Manufacturing (4)
+Advanced Therapy Medicinal Products (1)
+Artificial Intelligence (1)
+Biotechnology (6)
+Commissioning & Qualification (6)
+Compounding (2)
+Containment (4)
+Critical Utilities (7)
+Data Integrity (18)
+Drug Shortages (3)
+GAMP® (17)
+Good Manufacturing Practice (2)
+Investigational Products (8)
+Knowledge Management (7)
+Lifecycle Management (6)
+Manufacturing Operations (10)
+Microbiological & Viral Contamination Control (16)
+Oral Solid Dosage (2)
+Packaging (3)
+Pharma 4.0™ (2)
+Process Analytical Technology (5)
+Project Management (6)
+Quality Assurance (9)
+Quality Control (2)
+Quality by Design (6)
+Regulatory (10)
+Single Use Technologies & Disposables (1)
+Sterile Products (2)
+Supply Chain Management (6)
+Sustainability (2)
+Sustainable Facilities, HVAC, & Controlled Environments (6)
+Validation (16)
+Professional Development Training
Combination Products: Challenges and Expectations Training Course
+Pharmaceutical Job Board
Featured Conferences