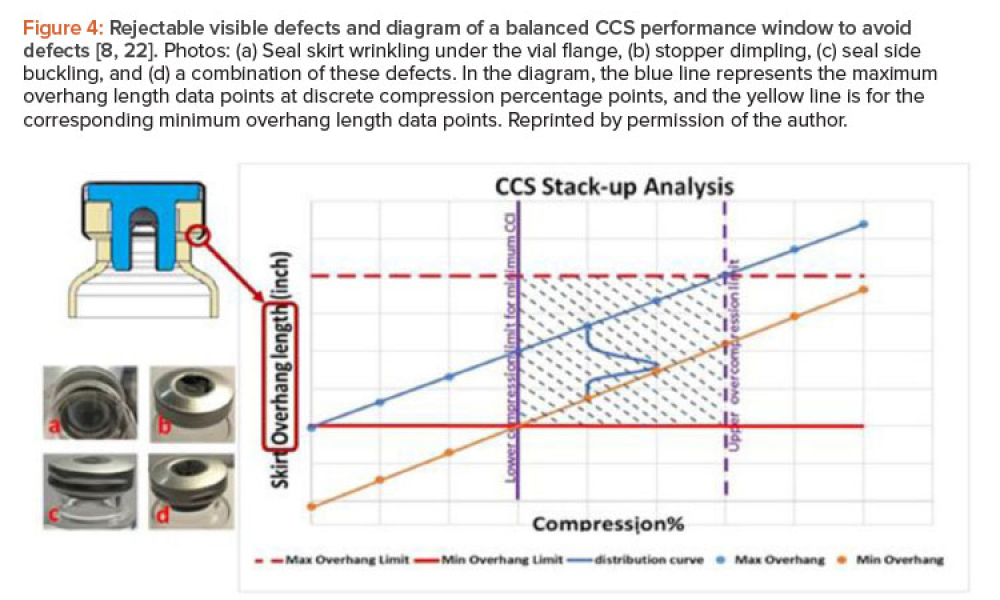
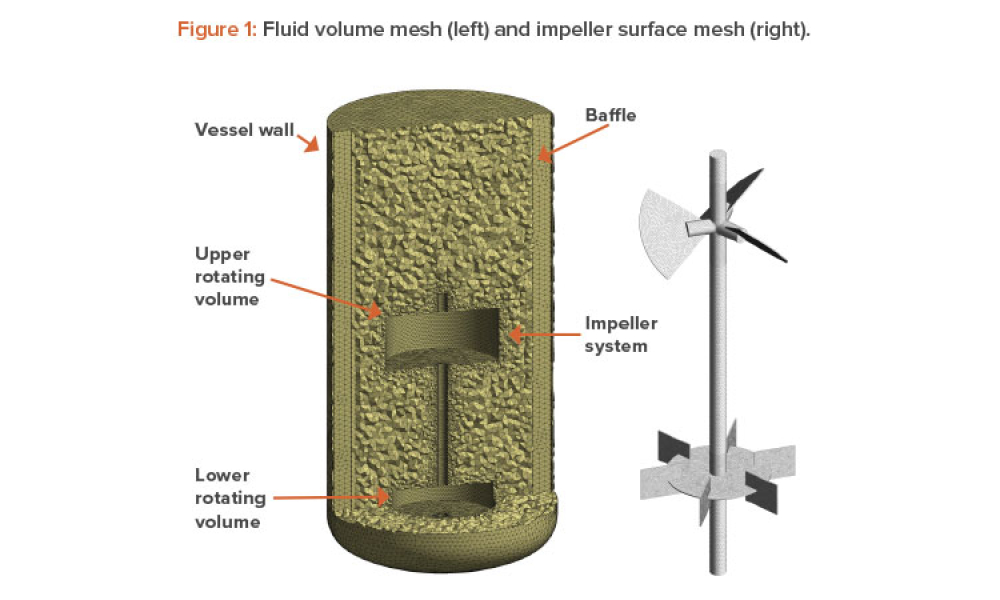
Sterile products processing relates to how sterile drug products are manufactured using aseptic (or free from contamination) process methods where the drug substance, excipients, and vehicle (e.g., saline or water for injection) are combined and filled into a container (such as a syringe)
Join the Conversation
As an ISPE member you can engage with 22 active CoPs, including new communities focused on Artificial Intelligence and Sustainability. Connect with experts and join the conversations: Become an ISPE member
ISPE members: Get more involved by volunteering.
Guidance Documents
Manufacturing Operations (1)
+Microbiological & Viral Contamination Control (1)
+Packaging (1)
+Sterile Products (2)
+Community Discussions
Community Discussions
Oct 04, 2025
Validation
Oct 01, 2025
Sustainable Facilities, HVAC, & Controlled Environments
Packaging
Oct 01, 2025
Information Systems
Data Integrity
GAMP®
Oct 01, 2025
Validation
Sep 30, 2025
Sep 26, 2025
Validation
Sep 25, 2025
Validation
Webinars
Upcoming
On-Demand
iSpeak Blog Posts
Pharmaceutical Engineering® Magazine
Videos
Professional Development Training
Aseptic Processing & Annex 1 Training Course
+White Papers
September / October 2025
As regulatory trends and quality initiatives continue to evolve in the pharmaceutical industry, this…
July / August 2025
The AI Paradox Cover: The increasing digitalization of the pharmaceutical and medical device…
May / June 2025
The ATMP Issue: In this issue, we focus on the manufacturing of advanced therapy medicinal products…