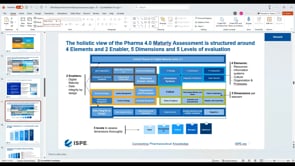
ISPE and its members are developing the roadmap to introduce Industry 4.0, also referred to as the Smart Factory, to the pharmaceutical industry as Pharma 4.0™.
Mission Statement
 The aim is to provide practical guidance, embedding regulatory best practices, to accelerate Pharma 4.0™ transformations. The objective is to enable organizations involved in the pharmaceutical product lifecycle to leverage the full potential of digitalization to provide faster therapeutic innovations and improved production processes for the benefit of patients.
The aim is to provide practical guidance, embedding regulatory best practices, to accelerate Pharma 4.0™ transformations. The objective is to enable organizations involved in the pharmaceutical product lifecycle to leverage the full potential of digitalization to provide faster therapeutic innovations and improved production processes for the benefit of patients.Implementing new Industry 4.0-based manufacturing concepts in Pharma 4.0™ requires alignment of expectations, definitions, and interpretation, with global pharmaceutical regulations.
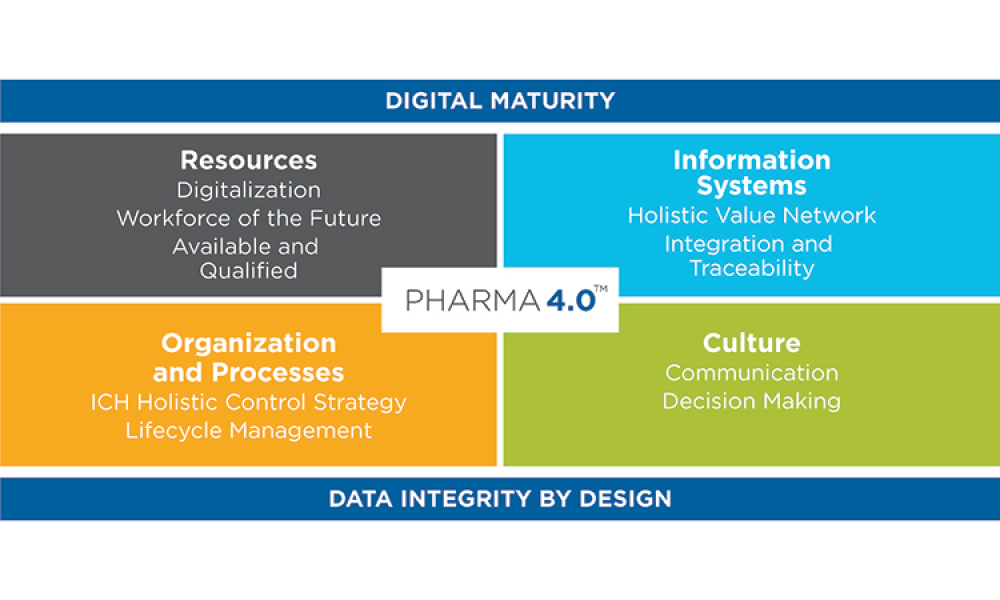
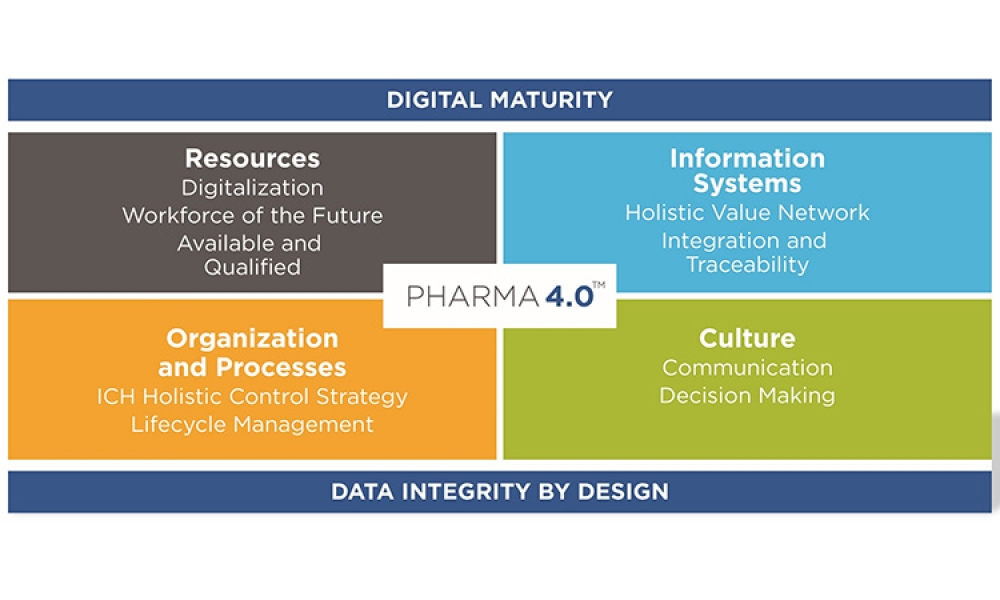
While Industry 4.0 has been called a new industrial revolution, Pharma 4.0™ implementation will more likely resemble an evolution in which digitalization and automation meet very complex product portfolios and cycles. It is therefore important to achieve an accepted understanding of readiness and maturity, starting with additional digital enablers and elements added to the ICH Q10: The Pharmaceutical Quality System along the product life cycle. It is also important to develop business cases to showcase which Industry 4.0 automation and digitalization technologies can be applied to the pharmaceutical industry and what implications are faced due to the increasingly complex regulatory challenges in the pharmaceutical and biotechnology industries.
Digitalization, an important component of Pharma 4.0™, will connect everything, creating new levels of transparency and adaptivity for a “smart” plant floor. This will enable faster decision-making, and provide in-line and on-time control over business, operations, quality, and regulatory compliance. Notably, this new connectedness will require higher levels of security, since linked systems heighten vulnerability.
Join the Conversation
As an ISPE member you can engage with 22 active CoPs, including new communities focused on Artificial Intelligence and Sustainability. Connect with experts and join the conversations: Become an ISPE member
ISPE members: Get more involved by volunteering.
Guidance Documents
Advanced Manufacturing (1)
+Data Integrity (1)
+Pharma 4.0™ (2)
+Process Analytical Technology (1)
+Community Discussions
Community Discussions
Jul 08, 2025
Pharma 4.0™
Apr 16, 2025
Information Systems
Regulatory
Advanced Manufacturing
Artificial Intelligence
Apr 07, 2025
Advanced Manufacturing
Artificial Intelligence
Mar 28, 2025
Information Systems
Regulatory
Advanced Manufacturing
Artificial Intelligence
Mar 14, 2025
Advanced Manufacturing
Critical Utilities
Nov 04, 2024
Information Systems
Management
Pharma 4.0™
Project Management
Pharmaceutical Engineering Magazine Articles
Professional Development Training
Good Engineering Practice (GEP) Training Course
+ICH Q7: API Guidelines
+ICH Q8: QbD in Manufacturing
+ICH Q10: Pharmaceutical Quality System (PQS)
+A GAMP® Guide to Computerized Systems Compliance
+Videos
iSpeak Blog Posts
Featured Conferences

Webinars
Upcoming
On-Demand
White Papers
May / June 2025
The ATMP Issue: In this issue, we focus on the manufacturing of advanced therapy medicinal products…
March / April 2025
A Skill Management Framework for a Pharma 4.0™ Workforce Feature: Pharma 4.0™ is driving fundamental…
January / February 2025
GAMP® is indispensable for safeguarding the safety, quality, and compliance of pharmaceutical…