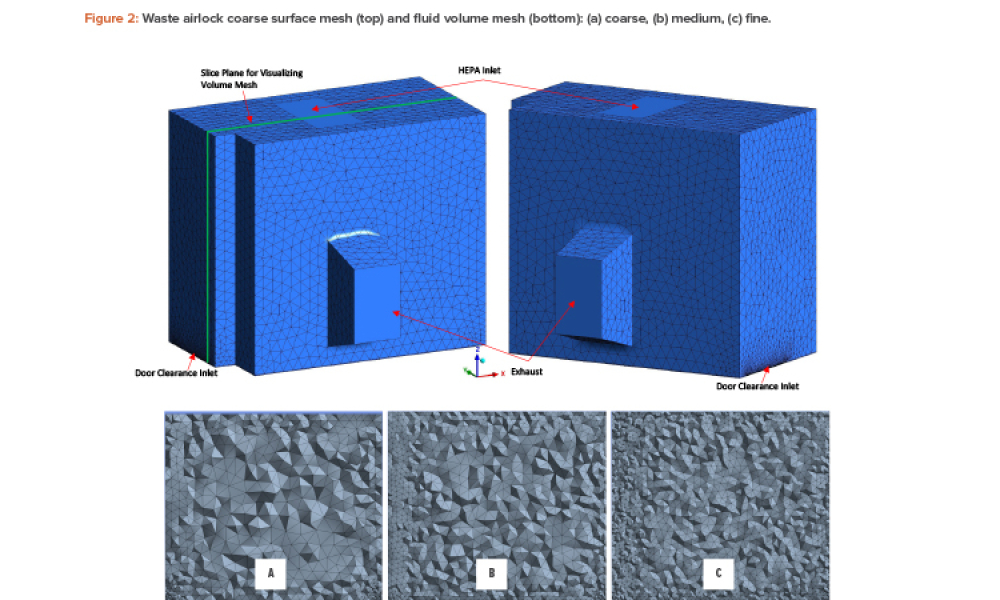
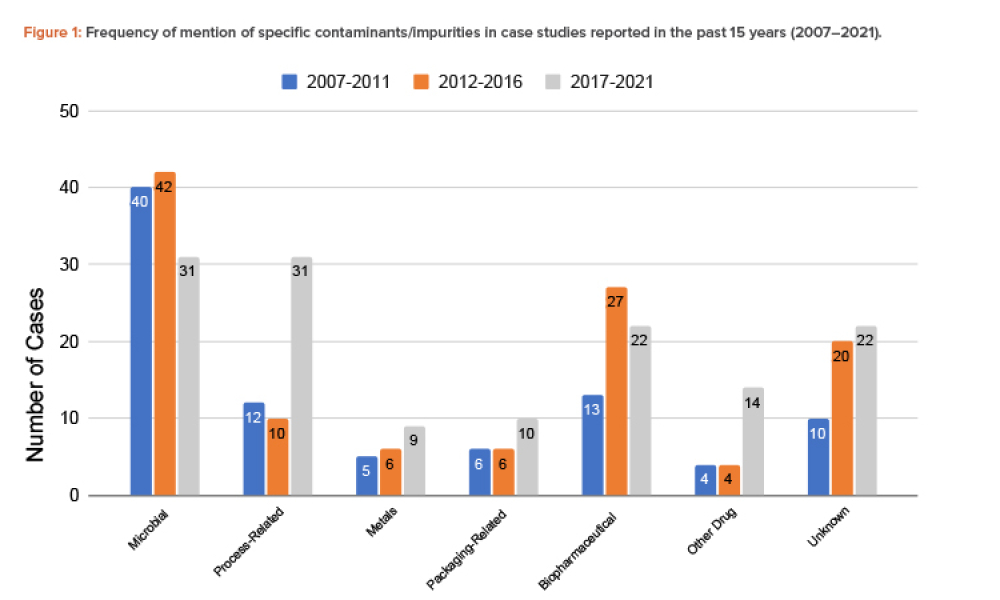
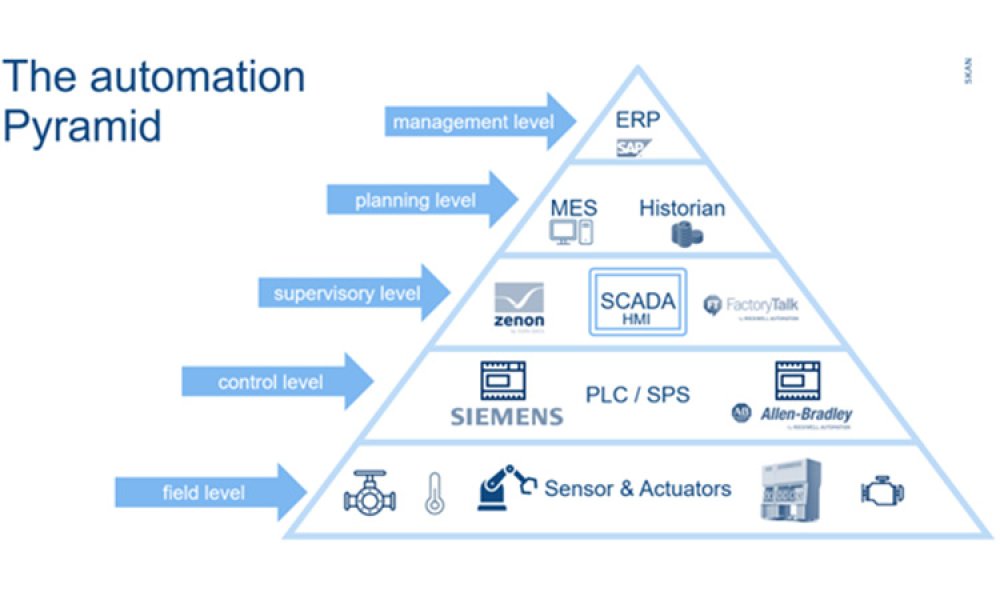
Manufacturing operation means a process in which materials are changed, converted, or transformed into a different state or form from which they previously existed and includes refining materials, assembling parts, and preparing raw materials and parts by mixing, measuring, blending, or otherwise committing such materials or parts to the manufacturing process. "Manufacturing operation" does not include packaging.
Join the Conversation
As an ISPE member you can engage with 22 active CoPs, including new communities focused on Artificial Intelligence and Sustainability. Connect with experts and join the conversations: Become an ISPE member
ISPE members: Get more involved by volunteering.
Guidance Documents
Advanced Manufacturing (2)
+Advanced Therapy Medicinal Products (1)
+Biotechnology (3)
+Data Integrity (1)
+Facilities of the Future (1)
+GAMP® (1)
+Good Manufacturing Practice (7)
+Lifecycle Management (1)
+Manufacturing Operations (18)
+Microbiological & Viral Contamination Control (3)
+Oral Solid Dosage (2)
+Regulatory (1)
+Sterile Products (1)
+Sustainability (1)
+Pharmaceutical Engineering Magazine Articles
Webinars
Upcoming
On-Demand
iSpeak Blog Posts
Professional Development Training
ISPE Hands-On Controlled Production of Drug Substance and Drug Product in Biomanufacturing
+GxPs for Leadership
+GMP Fundamentals: Production & Process Controls
+GMP Fundamentals: Buildings and Facilities
+Airflow Pattern Visualization (AFPV)
+Critical Utility GMP Compliance
+HVAC & Environmental Control for Life Science Facilities Training Course
+Storage, Delivery, and Qualification Pharma Water Training Course
+GMP Sterile Pharmaceutical Manufacturing Facility Training Course
+Pharmaceutical Water Systems
+Videos
White Papers
July / August 2025
The AI Paradox Cover: The increasing digitalization of the pharmaceutical and medical device…
May / June 2025
The ATMP Issue: In this issue, we focus on the manufacturing of advanced therapy medicinal products…
March / April 2025
A Skill Management Framework for a Pharma 4.0™ Workforce Feature: Pharma 4.0™ is driving fundamental…