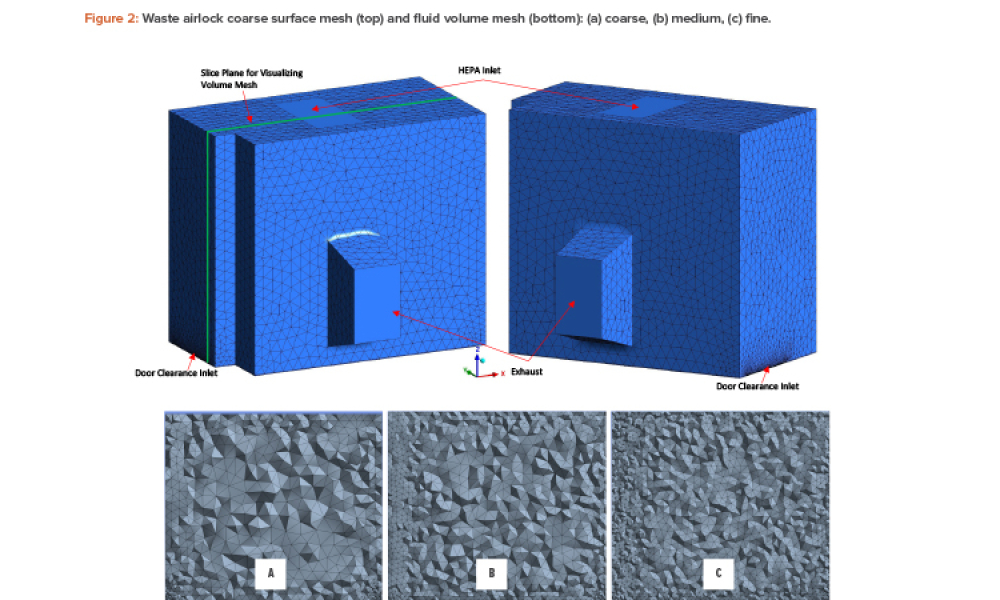
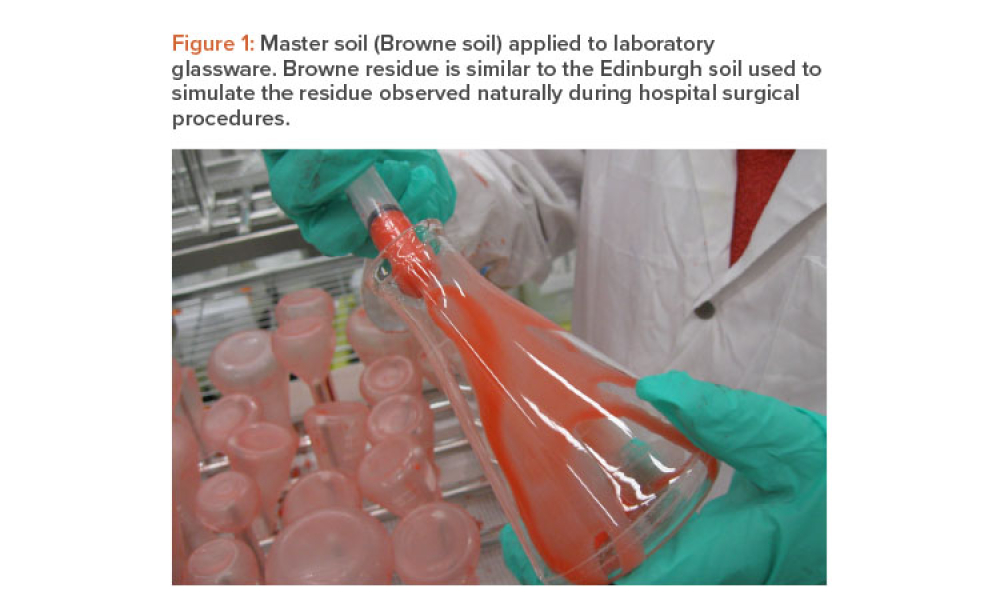
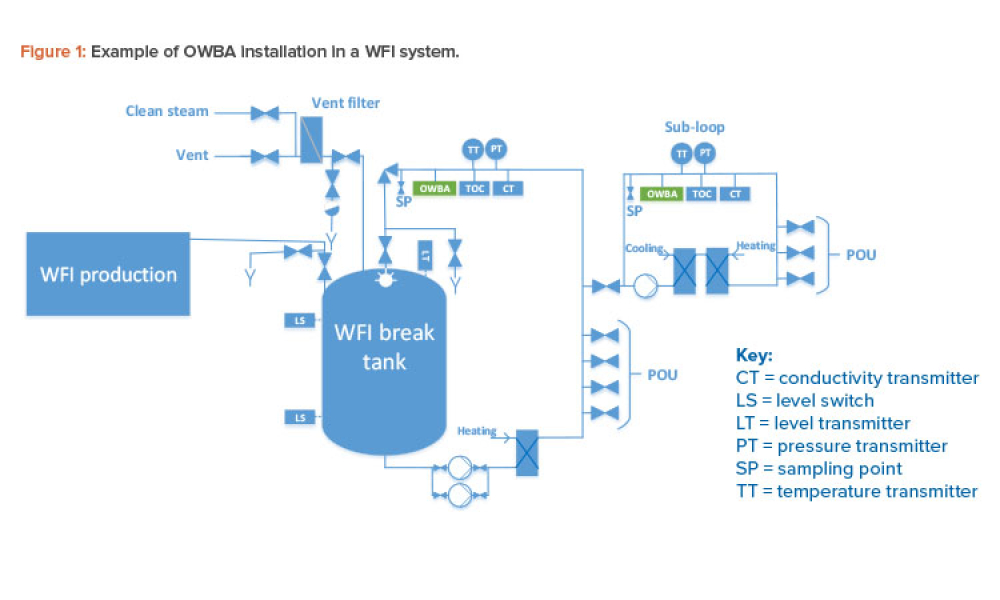
Quality Control (QC) is a detailed system of inspection and control covering the production, evaluation, and distribution of pharma drugs a manufacturer produces.
Join the Conversation
As an ISPE member you can engage with 22 active CoPs, including new communities focused on Artificial Intelligence and Sustainability. Connect with experts and join the conversations: Become an ISPE member
ISPE members: Get more involved by volunteering.
Guidance Documents
Critical Utilities (1)
+Microbiological & Viral Contamination Control (2)
+Quality Control (2)
+Community Discussions
Community Discussions
Apr 16, 2025
Information Systems
Regulatory
Advanced Manufacturing
Artificial Intelligence
Mar 28, 2025
Information Systems
Regulatory
Advanced Manufacturing
Artificial Intelligence
Nov 04, 2024
Regulatory
Supply Chain
Active Pharmaceutical Ingredients
Good Manufacturing Practice
Oct 31, 2024
Regulatory
Quality
Good Manufacturing Practice
Sustainable Facilities, HVAC, & Controlled Environments
Webinars
Upcoming
On-Demand
iSpeak Blog Posts
Pharmaceutical Engineering Magazine Articles
Videos
Professional Development Training
ICH Q6A: Specifications, Test Procedures, and Acceptance Criteria for New Drug Substances and Products
+GMP Fundamentals for the Pharmaceutical Industry
+GMP Auditing for Quality Assurance Training Course
+White Papers
July / August 2025
The AI Paradox Cover: The increasing digitalization of the pharmaceutical and medical device…
May / June 2025
The ATMP Issue: In this issue, we focus on the manufacturing of advanced therapy medicinal products…
March / April 2025
A Skill Management Framework for a Pharma 4.0™ Workforce Feature: Pharma 4.0™ is driving fundamental…