GMP refers to the Good Manufacturing Practice regulations promulgated by the US Food and Drug Administration under the authority of the Federal Food, Drug, and Cosmetic Act (See Chapter IV for food, and Chapter V, Subchapters A, B, C, D, and E for drugs and devices.) These regulations, which have the force of law, require that manufacturers, processors, and packagers of drugs, medical devices, some food, and blood take proactive steps to ensure that their products are safe, pure, and effective.
GMP regulations require a quality approach to manufacturing, enabling companies to minimize or eliminate instances of contamination, mixups, and errors. This protects the consumer from purchasing a product which is not effective or even dangerous. Failure of firms to comply with GMP regulations can result in very serious consequences including recall, seizure, fines, and jail time.
GMP regulations address issues including record keeping, personnel qualifications, sanitation, cleanliness, equipment verification, process validation, and complaint handling. Most GMP requirements are very general and open-ended, allowing each manufacturer to decide individually how to best implement the necessary controls. This provides much flexibility, but also requires that the manufacturer interpret the requirements in a manner which makes sense for each individual business.
GMP is also sometimes referred to as "cGMP". The "c" stands for "current," reminding manufacturers that they must employ technologies and systems which are up-to-date in order to comply with the regulation. Systems and equipment used to prevent contamination, mixups, and errors, which may have been first-rate 20 years ago may be less than adequate by current standards.
Join the Conversation
As an ISPE member you can engage with 22 active CoPs, including new communities focused on Artificial Intelligence and Sustainability. Connect with experts and join the conversations: Become an ISPE member
ISPE members: Get more involved by volunteering.
Pharmaceutical Engineering Magazine Articles
Guidance Documents
Active Pharmaceutical Ingredients (1)
+Compounding (1)
+Good Manufacturing Practice (9)
+Manufacturing Operations (7)
+Quality Assurance (1)
+Regulatory (1)
+Community Discussions
Community Discussions
Jul 24, 2025
Information Systems
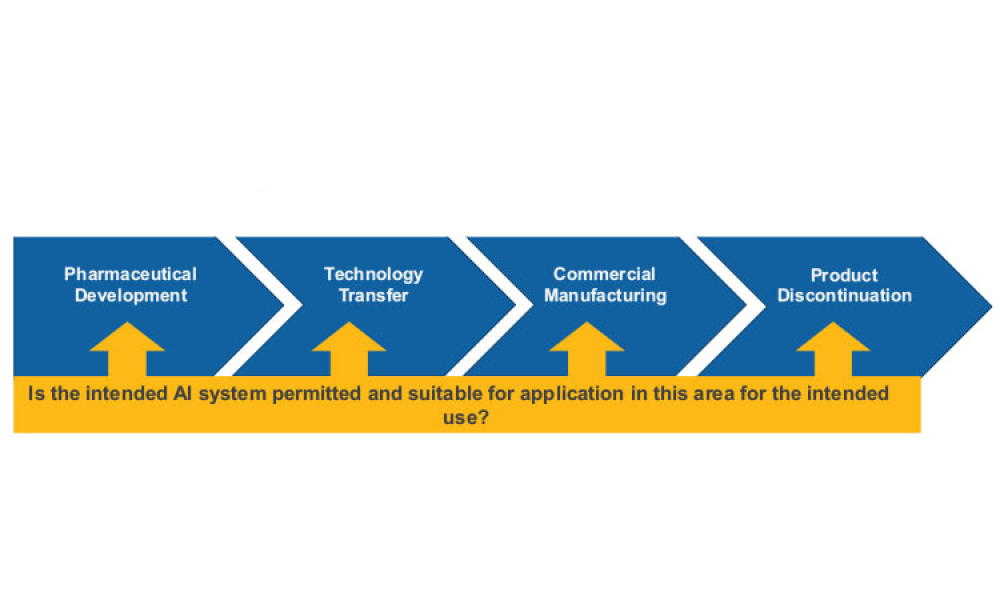
Artificial Intelligence
Data Integrity
Apr 16, 2025
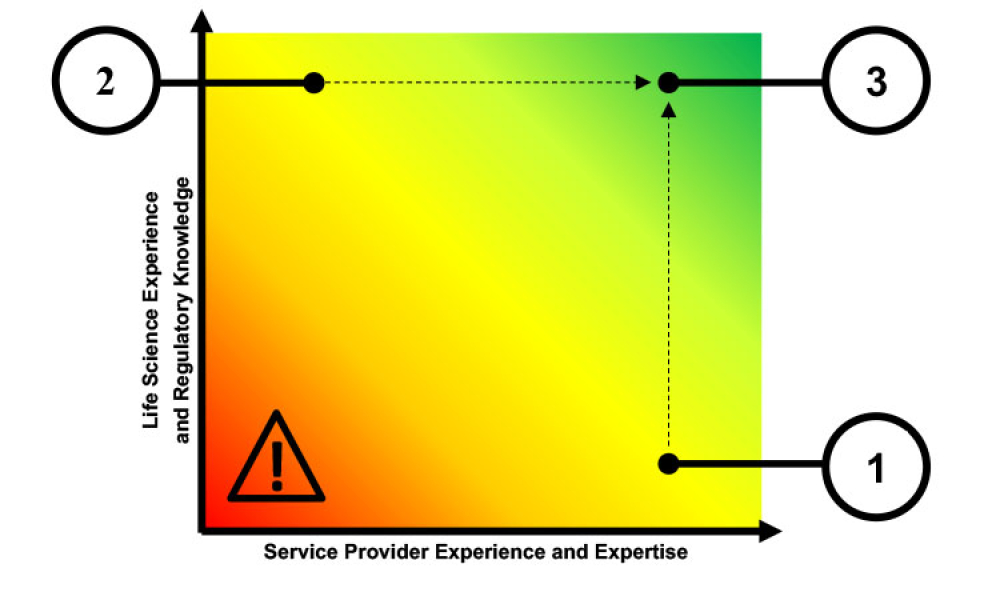
Information Systems
Regulatory
Advanced Manufacturing
Artificial Intelligence
Mar 28, 2025
Information Systems
Regulatory
Advanced Manufacturing
Artificial Intelligence
Jan 27, 2025
Good Manufacturing Practice
Sustainable Facilities, HVAC, & Controlled Environments
Dec 04, 2024
Good Manufacturing Practice
Nov 04, 2024
Regulatory
Supply Chain
Active Pharmaceutical Ingredients
Good Manufacturing Practice
Oct 31, 2024
Regulatory
Quality
Good Manufacturing Practice
Sustainable Facilities, HVAC, & Controlled Environments
Webinars
Upcoming
On-Demand
White Papers
September / October 2024
PIC/S in Latin America: Harmonization of cGMP Procedures Cover: This article offers an overview of…
GMP Regulation Handbook: General, Finished Pharmaceuticals, 21 CFR Parts 210 & 211
Regulation Handbook: 21 CFR Parts 210 (General) & 211 (Finished Pharmaceuticals) Current Good…
GMP Regulation Handbook: Medical Devices, 21 CFR Part 820
Regulation Handbook: 21 CFR Part 820: Medical Devices Quality Systems Regulation (formerly known as…
GMP Regulation Handbook: Electronic Signatures, 21 CFR Part 11
The 21 CFR Part 11 regulation handbook is used in association with ISPE training courses. Attendees…
GMP Regulation Handbook: Dietary Supplements, 21 CFR Part 111
GMP Regulation Handbook 21 CFR Part 111 Code of Federal Regulations US Food and Drug Administration…

iSpeak Blog Posts
Featured Conferences