Pharma 4.0™ Is Gaining Momentum and Driving Innovation

The fifth Pharma 4.0™ conference was held December 2022 in Vienna, Austria, in combination with the Aseptic Processing conference. Nearly 500 participants attended either in person or online to learn about the latest developments.
The concept of Industry 4.0 has taken on significantly increasing momentum in the pharmaceutical industry. Pharma 4.0™ is now probably the most important industrial approach or pharma manufacturing, particularly for sterile and aseptic products. The digital transformation of Pharma 4.0™ opens new opportunities for quality, safety, productivity, and patient benefit.
SmartFacturing
Michelangelo Canzoneri, Global Head of Group Smart Manufacturing at Merck KGaA, Darmstadt, Germany, opened his keynote “SmartFacturing” by asking how we should manage the new normal.
He referenced the “VUCA world,” which is recognized by:
- Volatility: Refers to the nature and dynamics of change, and the nature and speed of change forces and change catalysts. This asks, “What amount of change can we absorb before it negatively impacts us?”
- Uncertainty: Is linked to unpredictable demands, inventory, and capacity needs; new competitors entering the market; new government policies, etc. This asks, “What are potential signs of change? How fast can we respond to it?”
- Complexity: Is created by the magnitude of forces that confront companies. This asks, “How well do we understand the structures involved and their interdependencies?”
- Ambiguity: Is the potential for misinterpretation: the confusion of causes and effects. This asks, “What indicates that more information is needed before deciding?”
The framework conditions for this VUCA world have become more acute: Pandemics and geopolitical events are causing fragile and complex supply chains to collapse. Drug shortages are the consequence, seen in all regions of the world and possible for practically all pharmaceuticals.
If we focus on pharmaceutical operations, we see a major problem in the immense increase in the volume of data generated in connection with unit operations, e.g., in the recording of critical process parameters (CPPs), critical material attributes (CMAs), critical quality attributes (CQAs) needed for process understanding, and the control strategy. This drives the evolution from manufacturing to “smartfacturing.”
In the foundation, we detect what we consider “puzzle pieces”:
- Process technologies: production, purification, formulation, fill and finish, systems and flexware, unit operation “connectology,” media development, and formulation solutions
- Process analytical technologies: chemometric in-line, at-line, and online sensors; sampling to enable at-line testing; monitoring; automated sample preparation; multivariate data analysis and design of experiments; and process control
- Digital technologies: cloud, machine learning, data processing and cleaning, deep learning, feature extraction and engineering, natural language processing, predictive modeling, blockchain, and digital twins.
In the convergence, we detect a “picture” of autonomous manufacturing, quality by design, cognitive supply chain, and plug and produce. The cross-functional frame conditions are quality and regulatory, cybersecurity, end-to-end data management, and standards. The expected benefits include reduced time to market, decreased cost for capital expenses (CAPEX), and decreased cost of goods sold (COGS) while operating expense (OPEX), sustainability, and quality increase. In fact, the smart factory framework comprises 20 dimensions (see Figure 1).

When developing a roadmap for smartfacturing (see Figure 2), it should be considered that 80% of manufacturers are somewhere between level 1 and 2, and only a few have reached level 3.
At the beginning of a roadmap development there are always two main conditions. The first is maturity assessments for structural capabilities, performance, quality, and data/digital. This must be harmonized with the second condition: corporate strategy and business priorities. Building a good roadmap requires careful prioritization and mapping of interventions. Key business user stories may help build scenarios of a digital journey and lead to one IT architecture and ontologies.
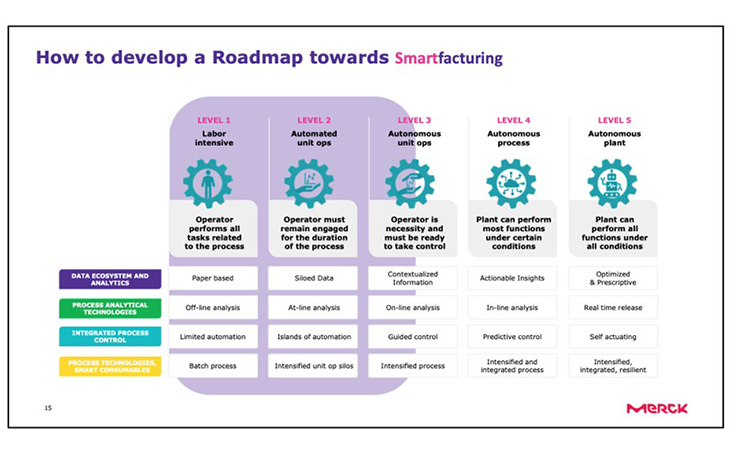
In summary, the following steps from manufacturing to smartfacturing are meaningful:
- Define your enterprise vision.
- Define your business specific strategy, priorities, and methodology.
- Define and execute your plant-specific roadmaps.
- Create a focused, single smart manufacturing capability portfolio linked to digital and quality maturity assessments and plant KPIs.
Where Are We with Pharma 4.0™?
Christian Wölbeling, Körber Pharma, Germany, gave an overview addressing where we are with Pharma 4.0. Pharma 4.0™ started in 2015 at the ISPE Germany/Austria/Switzerland (D/A/CH) “Plug and Produce” workshop in Basel, Switzerland. Then in 2015, ISPE in Europe founded the Special Interest Group (SIG) Pharma 4.0™, driven by the recognition that digital transformation offers a big chance to finally realize the US FDA’s Pharmaceutical Quality for the 21st Century Initiative to the full extent.
Based on the FDA’s vision, the Pharma 4.0™ SIG added the digital dimension:
“We provide practical guidance, embedding regulatory best practices, to accelerate Pharma 4.0 transformations. Our objective is to enable organizations involved in the product lifecycle to leverage the full potential of digitalization to provide faster innovations for the benefit of patients.”
Another solid basis for Pharma 4.0™ concepts are the ICH guidelines, primarily Q10, but also Q7 to Q12. An operating model was developed, which shows the elements and enablers for Pharma 4.0™ (Figure 3).
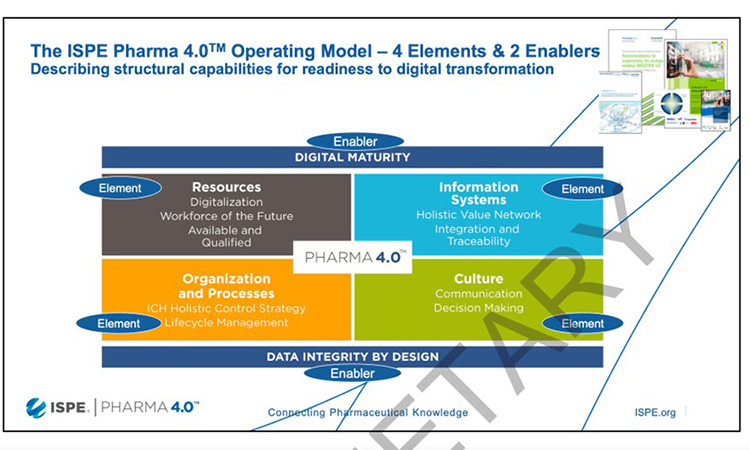
Working groups were created for the following topics: impact and maturity models, plug and produce, continuous process verification (CPV) and process automation, process maps and critical thinking, holistic digital enablement, validation 4.0, and management communication strategy.
In the years leading up to 2022, numerous publications were issued; networking events, webinars, and conferences were held; and regulatory panel discussions with regulators performed. Pharma 4.0™ hackathons were established, and the ISPE Factory of the Year (FOYA) award category was created.
ISPE Pharma 4.0™ went global and in 2022 the SIG developed into a Community of Practice (CoP). The next important milestones for 2023 are the ISPE Pharma 4.0™ Baseline Guide, followed by trainings and maturity assessments, and finally good practice guides to follow.
Pharma 4.0™ should tie to the new ISPE Quality Metrics & Advanced Pharmaceutical Quality (APQ) Guidelines, which consider quality maturity first and then digital maturity, and to ISPE GAMP®, which is important for maturity in computerized systems validation.
It’s also important to show the context around Pharma 4.0™ because this initiative is embedded in the pharmaceutical healthcare system. A main area to consider is connections, such as:
- Bridging industry 4.0 to a pharma-specific Pharma 4.0™ concept
- Connecting regulators with industry and healthcare stakeholders, especially patients
- Bridging regional and national regulatory agencies, such as FDA, EMA, and Heads of Medicines Agencies (HMA) in Europe, and harmonizing with their ideas and concepts for the future
- Connecting departments within industrial organizations, particularly product development, commercial manufacturing, and engineering/information technology
Another area to consider is continuing growth. This includes ensuring maturity levels for all operating model elements follow the five general maturity levels defined by the FDA: 1–Initial, 2–Developmental, 3–Defined, 4–Managed, and 5–Optimized. Holistic thinking from all stakeholders for life-cycle management (from development to product phase-out) and end-to-end considerations (along the value network) should be encouraged. The central role of data management should be explained, noting that data must be structured, standardized, and interoperable to guarantee GMP-like data recovery, data transmission, and data contextualization (this is the basis for data-based decision-making in a big data world of digitalization). And finally, it should be acknowledged that the discussion of “why Pharma 4.0™” is becoming an increasingly rhetorical one, particularly in view of the growing momentum in the pharmaceutical industry and in various statements and white papers from key regulatory agencies.
The upcoming Pharma 4.0™ Baseline Guide will contain a collection of use cases for digital transformation, used technologies, and benefits, offering a sound response to the “why” question.
Setting the Stage for eData Exchange
Stephen Wing, Head of Analytical and Logistical Services, Life Science Business, Merck KGaA, Germany spoke about how we can set the stage for eData exchange.
An eData platform can eliminate errors and save time; reduce process variability; enable next-generation biologics manufacturing, predictive analytics, and advanced process monitoring and control; and improve process and operational efficiency.
The proposed development strategy is to integrate supplier raw material data into corporate enterprise resource planning (ERP) and quality systems and to transfer data into a structured and standardized form respecting the regulatory requirement for data integrity (see Figure 4). So prepared, eData can be used in a process monitoring system covering all manufacturing and quality databases.

The targeted analytical and logistical solutions are shown in Figure 5. All available quality and logistics data can be used because they are interoperable due to standardization. Trends in quality and manufacturing data can be used for predictive analysis of the outcome. The connection to logistics data can ensure that the raw material quality can be adapted in time to avoid later deviation or aberrative trends in production.
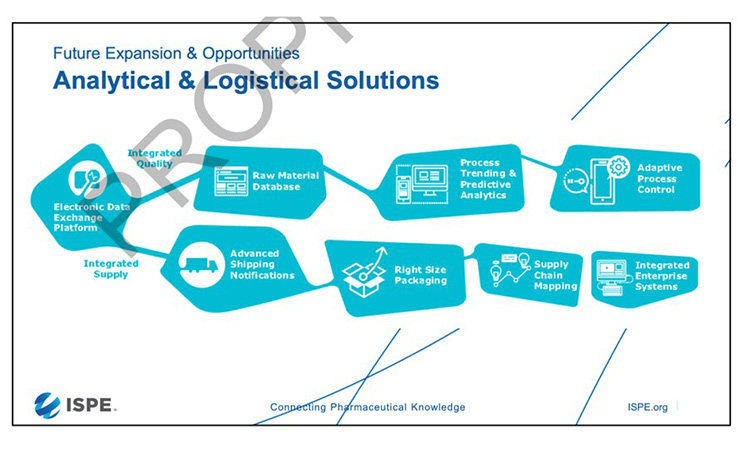
However, such a project will face some major barriers, such as lack of digital maturity, disparate legacy systems, lack of industry standards, lack of management supply, cost of installation, security concerns, and, finally, cultural hurdles ending in lack of collaboration, though none of these are unmanageable.
Pharma 4.0™ and Environmental Safety Governance
Joydeep Ganguly, SVP Corporate Operations, Gilead Sciences Inc., raised the question, “Is Pharma 4.0 the answer to bridging operational excellence and environmental safety governance (ESG) ambitions?” Ganguly considers Pharma 4.0™ principles as a catalyst for ESG.
Thousands or millions of queries and results between AI engine and data form biased decisions. Biased decisions and hundreds of thousands or millions of decisions make a large “splash.”
Use cases in the ESG area include reducing waste, utilizing resources, and ensuring supplier diversity.
Reducing waste
- Reducing energy leakage in energy-intensive fume hoods: analysis of face velocity, air flow, time, and sash positions creates an email alert to user when parameters met
- Reducing waste via prediction of root cause inefficiencies: using the power of predictive data science to find and root cause variances between actuals and forecast, down to equipment submeter
- Resource utilization identifying equipment performance issues: using the power of trend analysis to find and root cause equipment problem areas
- Resource utilization insights: using AI/ML powered optical sensors to identify utilization insights (ex, heat maps, trends, density averages and employee counts)
- Supplier diversity: worked with an emerging data analytics supplier to leverage AI/ML methods to help to create supplier options that are all “curated good choices.”
Digitization and Surveillance Expectations
Ronald Bauer, Head Institute, Surveillance, Agency for Health and Food Safety, Federal Office for Safety in Health Care, Vienna, Austria, addressed digitization and surveillance expectations. First, he considered EU GMP Annex 1: Manufacture of Sterile Medicinal Products and noted that risks should already be considered in the design of a manufacturing process. This includes:
- Monitoring systems (e.g., for processes through computerized systems or new forms of process control systems)
- Artificial intelligence (e.g., adaptive systems used in conjunction with computerized systems or process control systems), which evaluates generated data and makes decisions as part of these monitoring systems
- Robotics (e.g., automated docking of closures) to keep the human factor away from the product
Monitoring systems can be of varying complexity and may have varying levels of AI involvement. Simple systems can be automated decision trees (e.g., sensors) and complex systems can be learning neural networks (e.g., camera systems). A computerized system may control the sterilization process. For example, a control system can be automated in visual control, such as with optical inspection and particle detection. To ensure the AI is operating effectively, it’s critical that the data teaches the system to differentiate between cracks, fissure, fibers, parts of insects, etc.
Another area is represented by the revision of Annex 11, where digitalization will advance significantly in the future related to regulatory aspects in Annex 1. Surveillance authorities need to have regulatory approaches to evaluate new ways of digitalization.
The following are considerations when identifying the systems with more risk and the manufacturer’s integration approach:
- Do the risk assessment and a risk-based approach as the basis of qualification, validation, and operation reflect the continuous control strategy?
- Are GMP-critical elements of a system, that affect product quality and data integrity, adequately identified and emphasized in the validation approach?
- Is there a governance system for validation, data management, and data integrity?
The following are considerations for the manufacturer’s reliance on vendors:
- Are all GxP-relevant internal or external IT services assessed?
- Are hosted services as a black box not accepted?
- Is the vendors’ documentation complete in order to assess GxP risks and GxP compliance?
- Are the regulatory expectations of the “formal SLA” met (e.g., SaaS: the distribution and assessment of tasks demonstrated to inspectors)?
The following are considerations for producing and protecting electronic records:
- How well-defined is data management?
- Is the integrity of electronic data understood as a pivotal issue and underpinned by a holistic approach?
- Does the governance system of data integrity cover electronic data, paper-based data, and data migration?
- Is the governance system in line with continuous user management?
- Is the significance of networks and all relevant infrastructure considered?
- Does the interaction between the regulated user and the vendor ensure data integrity and is it sufficiently understood by both sides?
- Are all support processes regarding data identified and considered in validation (completeness of data sets in migration; backup, restore, archiving and the focus on the ability to restore for a given period)?
The following are considerations for artificial intelligence:
- The regulated user’s approach to development of the AI/ML
- Suitability of the model and model development (assessment of completeness and representativeness of training data, labeling of data with meta data, personnel qualification, documentation standard)
- Validation strategy as part of the risk-based integration into the process
- If relevant: adequate assessment of cloud solutions
- Verification of function during operation: does the model work, are malfunctions recognized, is the process under control?
Conclusion
The combined conference event brought discussion of these key Pharma 4.0™ and Annex 1 topics to the nearly 500 participants. The speakers delivered expert opinions, regulators views and use cases in the sessions that provided a wealth of information for an impactful event. There will be a follow up conference in December 2023 in Barcelona (Spain).




