In 2000, the U.S. Green Building Council (USGBC) introduced Leadership in Energy and Environmental Design (LEED) to create a methodology to promote sustainability-focused practices in the building industry. This article reviews the earliest protype that we know of for a LEED manufacturing facility, presents a real-world case study of a pharmaceutical manufacturing facility achieving LEED Gold,...

Downloads
This issue of Pharmaceutical Engineering looks at an array of sustainability topics.
Sustainability & the Life Sciences Industry: A Global Introduction
Cover: This introductory article surveys topics that will likely have a significant global impact on the way we conduct our business over the coming decade. We trace some of the history of sustainability in the life sciences industry and identify future issues of concern, including a number of areas where industry advancement cannot be made without true commitment to a full set of sustainable objectives.
Two Real-World Experiences in Global Sustainability
Feature: The article appraises the real-world experiences of two pharmaceutical companies approaching the rollout of energy- and water-reduction programs to selected facilities around the world. It is the result of more than two years of collaboration between the company corporate teams, individual site teams, and an external specialist consultant.
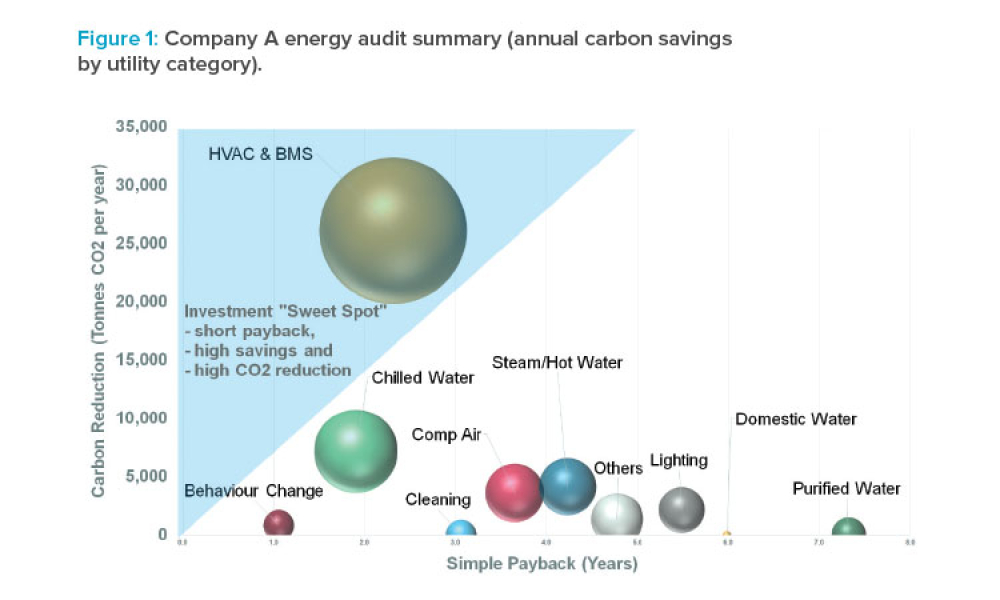
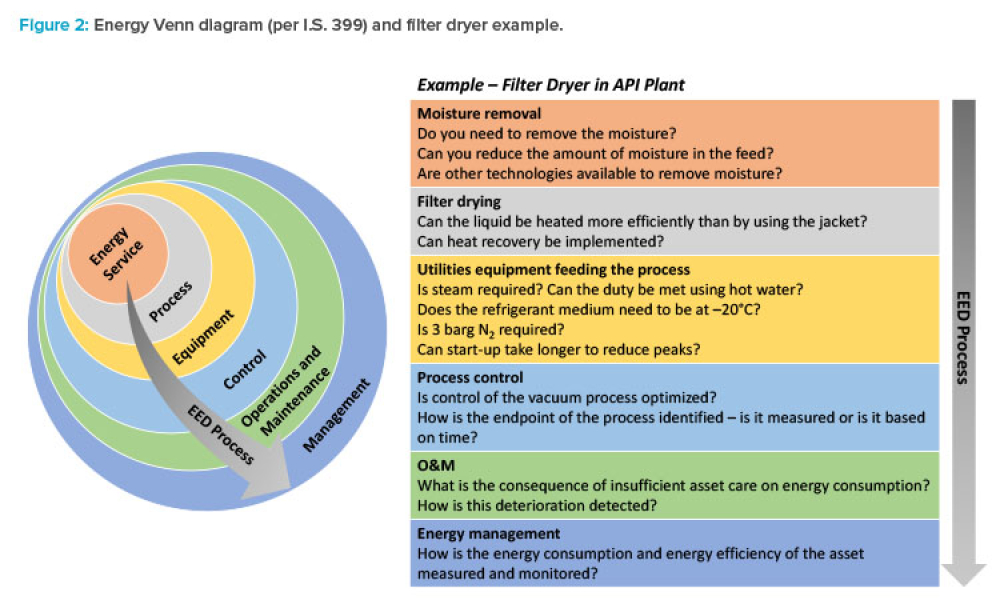
Implementation of a Formal Energy-Efficient Design Process
Feature: Sustainability is a key principle for pharmaceutical companies in 2020. However, translating corporate goals into meaningful improvements can be a challenge, particularly when competing factors such as complex technical requirements or ambitious project schedules are involved. This article looks at a process in use in Ireland and addresses the benefits of integrating this type of study into the design process.
Special Report: 2019 ISPE Annual Meeting & Expo:
-
Global Regulatory Town Hall: Answers to Key Questions
-
ISPE Global Hackathon 2019: Report from the Winning Team
In This Issue
We have some clarity over the desired end point of zero carbon within the next 30 years (or sooner for some pharma companies), but our industry is uncertain how we will achieve this objective. As pharma engineers, we will all play a role in delivering this end point through the continuous improvement of existing facilities and by designing, equipping, and building new facilities that will be...
The article appraises the real-world experiences of two pharmaceutical companies approaching the rollout of energy- and water-reduction programs to selected facilities around the world. It is the result of more than two years of collaboration between the company corporate teams, individual site teams, and an external specialist consultant.
Regulatory authorities have approved the use of recombinant monoclonal antibodies (mAbs) to treat infectious diseases
The 2019 ISPE Annual Meeting & Expo plenary featured a global regulatory town hall addressing a wide range of information about trends in regulations and other developments in the pharmaceutical industry....
The first ISPE Global Hackathon was held at the 2019 ISPE Annual Meeting & Expo with six teams and 36 participants, including 19 undergraduate students, 11 graduate students, and 6 Young Professionals...
As one of the larger and older ISPE Chapters, the ISPE Delaware Valley Chapter (DVC) is admired by other Chapters and Affiliates around the world.
Sustainability is a key principle for pharmaceutical companies in 2020. However, translating corporate goals into meaningful improvements can be a challenge, particularly when competing factors such as complex technical requirements or ambitious project schedules are involved.
The 2019 ISPE Max Seales Yonker Member of the Year Award was presented to Charlie Wakeham, a data integrity and compliance specialist based in Sydney, Australia, who has played an instrumental role in the development of GAMP®.
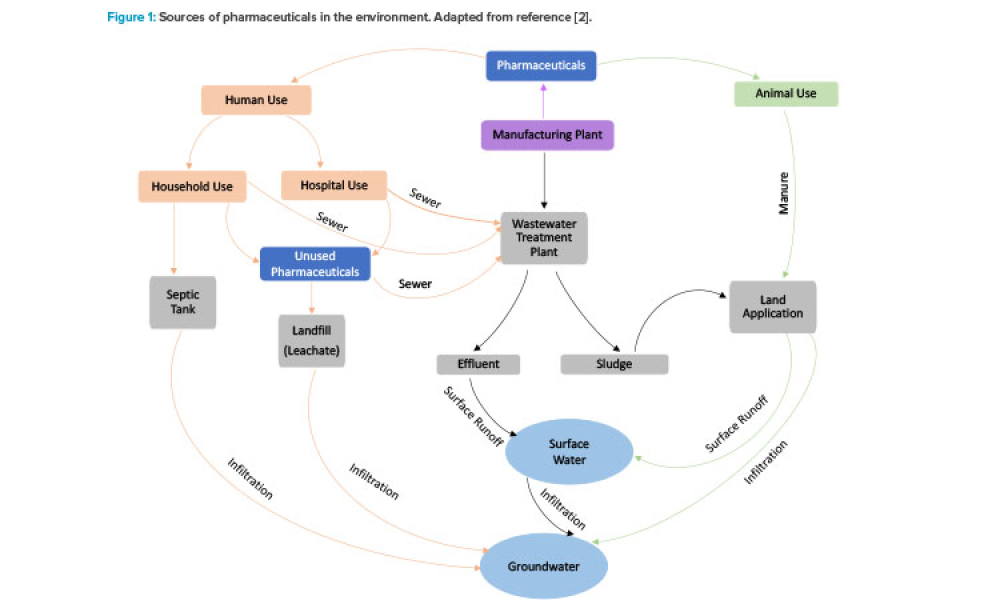
Medical treatments and pharmaceuticals are indispensable in improving quality of life. In recent years, however, pharmaceutical compounds have become a significant group of environmental pollutants, shown to pose risks to human health and have adverse environmental effects.
The 2019 ISPE Europe Pharma 4.0™ Conference held in Manchester, UK, on 20–21 November 2019 was attended by 300 participants, all of whom contributed to the growing momentum for
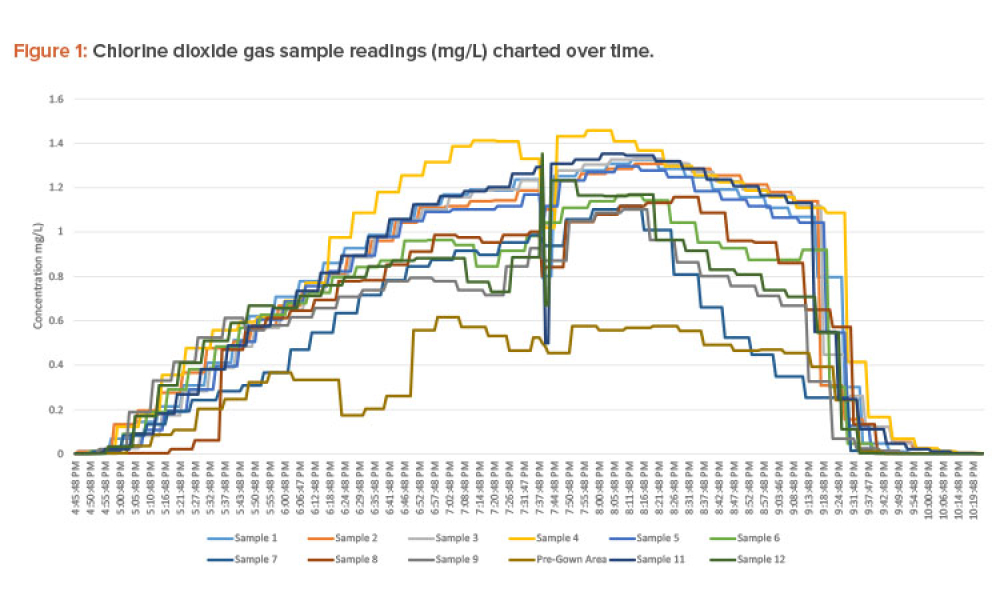
Manual decontamination procedures are laborious processes and can be costly, requiring significant time and resources to complete. Manual procedures also may need to be repeated if initial efforts do not fully kill pathogens. To reduce failures and potentially reduce cost, chlorine dioxide gas decontamination was investigated as an alternative solution.
I recently had dinner with a friend and colleague who was looking at taking on a new job role. She asked me what I thought about her salary request as part of this new opportunity. I asked, “Is that what you think you are worth?” She looked very confused by my response, and I realized that many of us do not often step back and determine our business value.
In this issue of Pharmaceutical Engineering®, we address an array of sustainability topics. This article surveys topics that will likely have a significant global impact on the way we conduct our business over the coming decade. We trace some history of sustainability in the life sciences industry and identify future issues of concern, including a number of areas...