Chlorine Dioxide Gas Decontamination vs. Liquid Disinfection
Manual decontamination procedures are laborious processes and can be costly, requiring significant time and resources to complete. Manual procedures also may need to be repeated if initial efforts do not fully kill pathogens. To reduce failures and potentially reduce cost, chlorine dioxide gas decontamination was investigated as an alternative solution.
The Bausch + Lomb (B&L) Vision Care production facility in Greenville, South Carolina, manufactures contact lens solutions in sterile processing areas within a clean environment. Because the manufactured products either clean contact lenses or are placed directly into a person’s eyes, they must be sterile and containers must be filled and sealed in an extremely high-quality environment.1
Each year, the facility closes for planned maintenance shutdowns. Though necessary, these shutdowns create unsterile environments because foreign equipment, tools, and people enter the clean areas. Therefore, the environment must be cleaned and disinfected before normal production resumes.
Manual Cleaning and Disinfection
Historically, manual cleaning and disinfection procedures to prepare the plant for reopening required nearly 100 personnel working in multiple shifts for over six days (three days to clean and then three days to disinfect rooms using mops and buckets). Rooms were cleaned with detergents and/or surfactants and then wiped down with a high-level disinfectant solution. To maintain high-quality standards, this cleaning and disinfection process has multiple stages: gross cleaning, followed by fine cleaning, followed by at least three rounds of disinfection. If any posttreatment swabs test positive for contaminants, that particular area might require additional treatment.
In general, manual cleaning and disinfecting activities use physical cleaning motions and disinfectants to kill organisms. Once cleaning is complete, a liquid disinfectant is used to disinfect the area. This cleaning process is considered effective at removing biological contaminants on environmental surfaces.
The disinfectant used is typically applied to a surface, a device surface, or a cloth. Once applied, the disinfectant sits for the contact time prescribed by its manufacturer.
The disinfectant used at the facility is a fast-acting, liquid cold sterilant/disinfectant, filtered through a 0.2-micron filter and specifically formulated for use in the sterilization and disinfection of hard environmental surfaces in pharmaceutical, medical device, biotech, and cosmetic manufacturing facilities. This product is a stabilized blend of peracetic acid, hydrogen peroxide, and acetic acid that provides fast, effective control of microbes, including spores. The disinfecting agent is typically used for a number of reasons: (a) ease of use; (b) consistent dilution because no mixing or activation is required; (c) efficacy—microbial control against bacteria, fungi, viruses, and bacterial spores; (d) safety—the low toxicity profile supports worker safety; (e) convenience—excellent material compatibility allows use on most environmental surfaces; and (f) flexibility and versatility of use—depending on the use concentration, contact time, and application method, the product can be used as a sterilant, sporicide, disinfectant, or sanitizer.
This process is costly and labor-intensive. The manufacturing facility consists of filling rooms, sterile staging areas, gowning areas, and sterile hallways, each with a significant amount of surface area. The filling lines and equipment have many surfaces to treat and thus require large amounts of the disinfectant solution. The company would spend approximately $150,000 to fully disinfect the entire sterile processing facility, and the disinfection process would take about three (24-hour) days and require a crew of nearly 100 people.
Gross Cleaning
Gross cleaning consists of scrubbing all stainless steel equipment with a cleaning solution and using brushes to remove all visible residue. Walls and ceilings are mopped, HEPA filters are wiped with an isopropyl alcohol (IPA)–soaked class 100 wipe, all returns are wiped with disinfectant-soaked lint-free towel, and floors are mopped with a disinfectant.
Fine Cleaning
Fine cleaning occurs after gross cleaning and consists of spraying a cleaning solution on all surfaces except HEPA filters and wiping all stainless steel surfaces and equipment, HVAC return vents, waste containers, curtains, plexiglass, and equipment. Some equipment is uninstalled to facilitate better cleaning. Walls and ceilings are mopped with the cleaning solution and floors are mopped with a disinfectant.
Disinfection
Prior to switching to gas decontamination, there were three rounds of disinfection. In the first round, everything was sprayed with a disinfectant solution, curtains and plexiglass were wiped with a disinfectant-soaked lint-free towel, and all walls and floors were mopped. The second round repeated the first round’s cleaning and included wiping the inside of some equipment hoppers as well. In the third round, all surfaces were sprayed and wiped with the disinfectant solution, and then all surfaces were wiped with an IPA-soaked class 100 wipe.
Once the cleaning/disinfection process was complete, the areas were swabbed to confirm the efficacy. If any area tested positive for contaminants, it had to be cleaned and disinfected again, increasing costs and requiring more time and effort.
Given the labor intensiveness, variability, lack of repeatability, and cost of the manual cleaning and disinfection process, B&L sought more efficient, reliable, and cost-effective alternatives. Chlorine dioxide gas was chosen as a test agent because it has been shown effective at decontamination of large-scale facilities,2, 3, 4 rooms and suites of rooms,5, 6, 7, 8, 9 isolators,10, 11, 12 processing vessels and tanks,13, 14 and biological safety cabinets.15, 16 See Table 1 for a comparison of manual disinfection and decontamination using chlorine dioxide gas.
| Manual Disinfection | Chlorine Dioxide Gas |
|
|---|---|---|
| Treatment time | 3 days (97 people) | 2 days (6 people) |
| Efficacy | Some positive swabs | All biological indicators dead; no positive swabs |
| Cost | ~$150,000* (~$100,000 in disinfectant solution + ~$50,000 in labor) |
$97,000 (all-inclusive) |
| Application method | Spray and wipe | Gassing |
| Method of kill | Oxidation | Oxidation |
| Level of kill | Sterilant | Sterilant |
Chlorine Dioxide Decontamination
Because chlorine dioxide is a true gas at room temperature (boiling point -40 °C), its distribution and penetration do not rely on an operator’s skill. As a gas, it reaches all areas—including cracks, crevices, and difficult-to-reach surfaces—and provides full coverage, making decontamination more successful than manual disinfection.
As the FDA states, “suitability, efficacy, and limitations of disinfecting agents and procedures should be assessed.”1 To do this, biological indicators (BIs) were placed throughout the space to test the process and ensure proper decontamination.
Gas Material and Equipment
- The following equipment was used to decontaminate the space:
- A 330,000 ft3 (9,344 m3) aseptic classified space
- Manual chlorine dioxide gas–generating systems (qty. 14)
- Chlorine gas cylinders (2% chlorine/98% nitrogen) (qty. 28)
- EMS chlorine dioxide gas–monitoring systems (qty. 2)
- Extension cords (100-feet and 25-feet; qty. 10 each)
- Blowers (approximately 1,800 CFM each; qty. 18)
- Small fans (qty. 40)
- Duct tape and plastic
- Spools of ¼-inch red polyethylene tubing (for gas injection; qty. 28)
- Spools of ¼-inch green polyethylene tubing (for gas sampling; qty. 10)
- Rolls of thin 3-mil plastic sheeting (for conveyor sealing; qty. 4)
- Roll of 6-mil plastic sheeting (for large-opening sealing; qty. 1)
- Low-level chlorine dioxide gas safety sensors (qty. 3)
- Pairs of BIs—106 Geobacillus stearothermophilus spore strips (qty. 20)
- Prepared culture media: formulated tryptic soy broth modified with pH indicator (qty. 20)
Sterile Processing Facility Decontamination
Gross and fine cleaning of the facility was completed as previously described prior to the chlorine dioxide gassing team’s arrival. Once cleaning was completed, decontamination followed in the ensuing steps.
Day 1—Arrival and Initial Setup
The decontamination team of five people arrived onsite in the early afternoon. The listed equipment was brought to the decontamination area, and the manual chlorine dioxide gas generators were set up outside the decontamination space. External windows and doors were taped and sealed to contain the gas during the actual decontamination process.
Day 2—Setup
The decontamination team arrived in the morning and split into smaller teams to continue sealing the space and setting up the decontamination equipment. Sealing began in the packaging transition area, which has small openings in walls where conveyors exit with finished product in sealed containers. These openings were sealed with a mixture of plastic and duct tape. Because of the nature of the facility, a special duct tape that leaves little to no residue was used. Sealing was performed on the outside surfaces so the sterilant would not miss important internal surfaces.
The HVAC for some areas was turned off, allowing roof units to be sealed. Some HVAC units were left on for workers’ comfort and to control humidity in the space.
Some HVAC units had exhaust and supply vents common with areas outside the cleanroom space. When gas enters ductwork, it will leak to outside areas unless the vents are sealed. Therefore, common vents outside the space were located and sealed with duct tape and plastic.
At the same time that the area was being sealed off, another team set up the gas generation system by evenly distributing blowers and small fans throughout the space. Blowers and fans were usually placed close to power outlets. Because the fans were used to speed up the diffusion of the gas and were not needed to force the gas into specific areas, where they were placed was not critical.
Red gas injection tubing was run from each generator to multiple locations within the space. Gas generators were located outside the space, ensuring that generators could easily be stopped if necessary for safety. Some gas injections points were combined in one area to minimize the time to place the tubing.
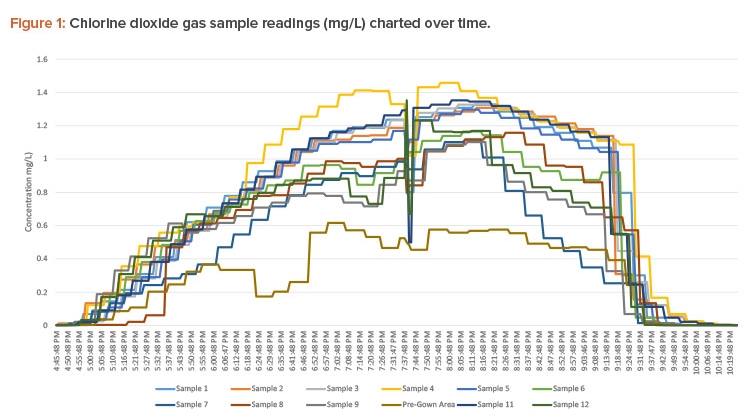
After the gas injection tubing was placed, the green tubing used for sampling gas concentrations was run from the chlorine dioxide gas–monitoring system’s gas sensor, which was placed outside the decontamination space, to locations in the space away from the gas injection sites. The monitoring system used a small diaphragm pump to draw in air samples from the different locations (one at a time) through a photometer to read the actual real-time chlorine dioxide concentration. The photometer measures the absorbance of the gas, and the monitoring system converts this absorbance into a chlorine dioxide gas concentration reading in mg/L. The monitoring system uses these readings to determine when the concentration reaches the required dosage.
In some projects, some areas may not come up to concentration as expected, either due to leakage or because gas consumption is greater than expected. If that happens, some generator injection points are moved to the spare injection points. Spare gas injection points were not used on this project.
Once the fans and tubing were set up, and most HVACs sealed, 20 pairs of BIs were placed at 20 locations throughout the facility to test the efficacy of the process. Pairs of BIs were used based on validation studies performed by Luftman and colleagues.15 In this study, it was decided that if both BIs were positive, the results were positive (growth); if both BIs were negative, results were negative (no growth). On the rare occasion that one BI was positive and one BI was negative, it was assumed, with a 95% confidence level, that there was a 5.7 log reduction of spores. For facility decontamination, these results would be considered successful and significantly more effective than utilizing a liquid disinfectant solution.
Once the BIs were placed, the remaining unsealed HVAC cooling coils were shut off, allowing outside humidity to enter the space and raise humidity in the room to over 70%. The decontamination took place during the summer months, so ambient/outside humidity was naturally high. Once room humidity was verified in all areas to be above 65% for a minimum of 30 minutes, the HVAC was shut down and sealed and then the last entry doorway was sealed.
Day 2—Gassing
At approximately 16:45 (4:45 p.m.), the gas cylinders were opened and gas injection began. Chlorine dioxide gas was generated by passing a low-level chorine gas (2% chlorine/98% nitrogen) through solid sodium chlorite cartridges, which converts the chlorine to a 99.9% pure chlorine dioxide gas. Workers walked around the decontamination space carrying low-level safety sensors to locate any possible leaks in any of the plastic and duct tape sealing. This task was performed periodically to ensure worker safety. Chlorine dioxide gas has a low odor threshold (0.1 ppm), which coincides with the 0.1 ppm, eight-hour personal exposure level.
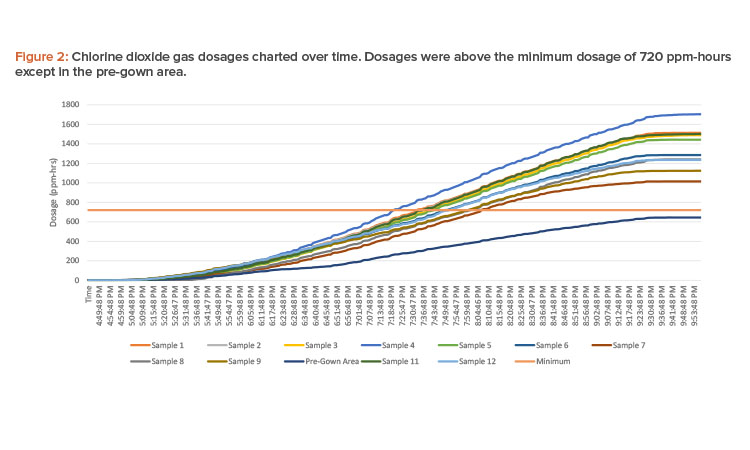
Gas injection ran continuously from 16:45 to 21:00 (4:45 p.m. to 9:00 p.m.) to accumulate a minimum dosage of 720 ppm-hours to achieve a 6-log reduction of spores (see Figure 1 for concentration readings and Figure 2 for dosages).
All concentrations were at or near the target of 1 mg/L, except for the pre-gown area (see Figure 1). This sample tubing had a leak that diluted the sample reading. The area was verified to be above concentration by visual inspection. A yellow-green color was observed inside the space, signifying the presence of chlorine dioxide gas. This inspection does not inform the user of the concentration; however, if the gas is highly visible, an experienced user knows the concentration is higher than the 1 mg/L target concentration.
After the dosage was reached, a team went up to the roof to unseal the air handling units (AHUs). At approximately 22:00 (10:15 p.m.), all AHUs were unsealed and turned on.
Aeration in the three sterile component staging areas was started at 21:00 (9:00 p.m.). These areas were identified to have no exhaust capabilities; therefore, a supplementary aeration system was set up in this area. This system consisted of four external blowers pulling air from the component stag-ing area and blowing it out the nearest rollup door to the plant exterior. All filling lines aerated in a normal amount of time. Safe levels of chlorine dioxide (0.1 ppm) were attained about 22:30 (10:30 p.m.) in all areas.
Around 23:00 (11:00 p.m.), three people entered the sterile facility and donned gowns following B&L procedures. The team removed the BIs, tub-ing, blowers, and fans and crated equipment. Then, the team used a low-level safety sensor to verify the gas concentration in all areas was below safe level. Once this was verified, all sealing plastic and tape were removed. The team exited the cleanroom at approximately 0:00 (12:00 a.m.). The remaining equipment was packed into the crates, and the team left the site at approximately 01:30 (1:30 a.m.). Finally, all BIs were incubated for 36 hours in the prepared culture media to test for growth. Table 2 lists the BI results.
Discussion
Many pharmaceutical/biotech companies operate cleanrooms, with some specified as sterile processing areas. This policy is to keep the product micro-biologically clean. During the normal course of events in cleanrooms, maintenance occurs. When maintenance occurs, contaminants can enter an area. To combat this, cleaning is performed after the planned service and before production is restarted. In the past, B&L used manual cleaning process (gross and fine) followed by three separate disinfecting steps.
The first part of any decontamination is cleaning to remove excess bioburden. Once this is accomplished, the decontamination step occurs. In the past, this was done at B&L by manually spraying and wiping the high-level disinfectant solution on all surfaces. Manual decontamination is not optimum because it is difficult for workers to spray and wipe every surface and get complete disinfectant coverage in the scratches, cracks, and crevices where organisms hide. When surfaces are sprayed with disinfectant, droplets are deposited onto the surface. If these droplets are larger than the cracks and crevices, they cannot penetrate completely. Even if the liquid disinfectant is fogged or mopped, it still does not reach every nook, crack, and crevice.
In contrast, chlorine dioxide, which is a true gas at room temperature, can penetrate every space due to its extremely small molecule size (0.124 nm10, 9). Compared to using liquids and a manual disinfection process, the advantages of gas decontamination become apparent.
| BI # | Location | Result After Incubation |
|---|---|---|
| 1 | FFS, valve lever | Negative |
| 2 | Line 7 machine in plastic enclosure | Negative |
| 3 | Line 5 valve on machine | Negative |
| 4 | Line 2a second door back left machine | Negative |
| 5 | Line 1 back round machine | Negative |
| 6 | Prep area center rack | Negative |
| 7 | CTA right window | Negative |
| 8 | Tote unload podium | Negative |
| 9 | Central sterile component staging center support | Negative |
| 10 | New area square support | Negative |
| 11 | Line 7 hallway machine | Negative |
| 12 | Line 6 angle beam in plastic enclosure | Negative |
| 13 | Line 6 hallway, center door machine | Negative |
| 14 | Line 5 hallway, center door machine | Negative |
| 15 | Line 4 machine back middle door | Negative |
| 16 | Line 2a hallway, machine | Negative |
| 17 | Line 1 hallway, machine middle door | Negative |
| 18 | Exit sanitization booth rack | Negative |
| 19 | Entry sanitization booth yellow bucket | Negative |
| 20 | Prep area 2 back right orange container | Negative |
| Positive control | Positive | |
Conclusion
The completed chlorine dioxide gas decontamination cycle at the B&L sterile processing facility was qualified as successful. All BIs were negative, apart from the positive controls.
The resulting ppm-hour dosage achieved from the decontamination cycle was adequate to provide a 6-log sporicidal reduction on the BIs after 36 hours of incubation. Total ppm-hour exceeded the required 720 ppm-hour for 6-log reductions of spores for all areas.
The decontamination cycle was also a success from an economic point of view: The costs of gassing were approximately 30% less than the traditional spray-and-wipe approach. With this cost saving, better coverage of the decontamination agent, and decreased downtime, this process was considered a complete success. B&L now uses chlorine dioxide gas as the preferred decontamination agent.




