The San Francisco Bay Area Chapter has won numerous ISPE Awards by virtue of their contributions to the pharmaceutical industry. They continue to have their finger on the pulse of the local industry and respond with innovative and educational programs. The Chapter’s Emerging Leaders Committee is...
Takeda Pharmaceuticals International AG is the 2021 Facility of the Year Awards Winner for Process Intelligence & Innovation for their F36 New Solid...
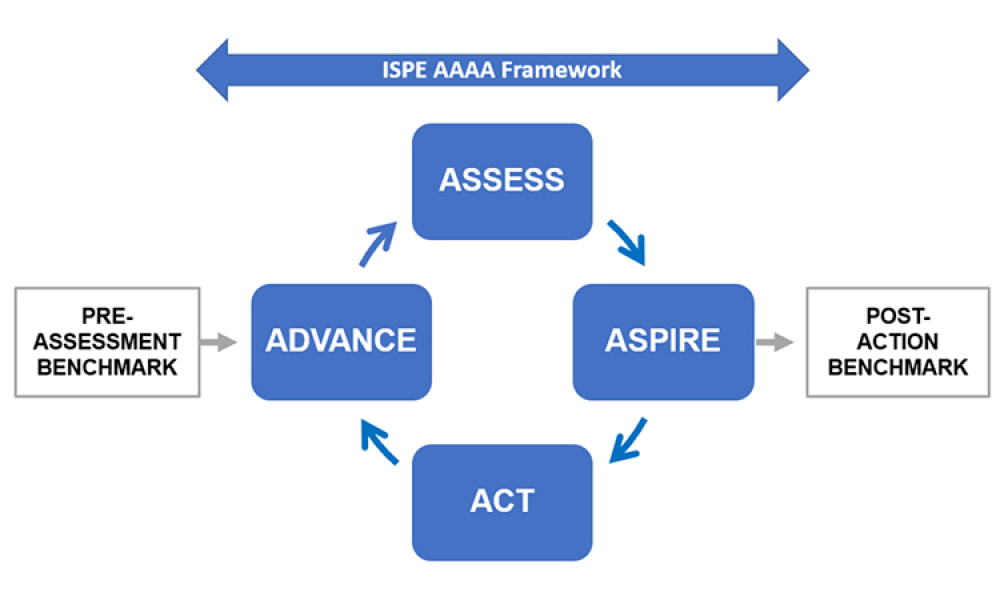
Does your company only focus on how to get the product to patient without considering what happens if product needs to be retuned?
Featured in this edition of iSpeak Reading Roundup, are the top blog posts from August 2021. Discover key insights for cleaning validation practices, risk-based approaches to quality, and more for what the pharmaceutical industry was reading last month.
ISPE is a global industry leader in scientific, technical, and regulatory advancement throughout the entire pharmaceutical lifecycle. As a result, ISPE is in a strong position and available to assist the US government, its allies, and like-minded regulatory partners with implementation of recommendations emerging from a report,
Imagine a time where medicines are made at your doorstep, where an illness can be treated in moments, storage conditions are a non-issue, quality is built in, and supply chains don’t matter. Your COVID-19 vaccine is manufactured, tested, and available for dosing, all within blocks of your home. Welcome to the age of transportable manufacturing.
ElevateBio is the 2021 Facility of the Year Awards Winner for Operational Excellence for their BaseCamp facility in Waltham, MA, USA.
It’s hard to believe we are more than halfway through 2021 and how 2020 is such a distance memory yet so ingrained into our minds it feels like it was yesterday. As COVID continues to be prevalent in society and with our continued focus on life saving medicines we as an industry must maintain our “Agility, Collaboration, Innovation”. The facility and engineering track would like to invite you...
Dr. Ferdinando E. Aspesi has been a member of ISPE for more than 29 years. Currently the Chair of the Pharmaceutical Engineering committee, he is also a member of the Delaware Valley Chapter and has been a member of the Global Pharmaceutical Manufacturing Leadership Forum, the PQLI Science and Technology Committee, and the Quality Metrics Team.
Takeda Pharmaceuticals International AG is the 2021 Facility of the Year Award Winner for Facility Integration for their Grange Castle P2 Facility in Grange Castle, Ireland.
This year’s ISPE Annual Meeting & Expo will be a combination of in-person presentations and events with virtual access to the presentations for those who cannot yet travel. The importance of information systems for our industry has evolved at an even faster rate. Digitalization is more important than ever, enabling us to work remotely effectively when needed and facilitates business...
All it takes is a quick Google search for the terms “carbon footprint and the pharmaceutical sector” to reveal some depressing reading. Results are led by a staggering piece of research from 2019 that states the global pharmaceutical industry is not only a significant contributor to global warming, but it also produces 13% more carbon emissions making medicines than car manufacturers do while...
I have always been a big advocate of mentoring – both being a mentor and a mentee. As great mentors frequently remind us, no one cares about your career more than you and you need to actively manage it. Mentors can help in numerous ways. One lesson I learned from mine is that networking is key to broadening knowledge, developing your career, and widening your circle of personal and...
I am very excited to be leading the Supply Chain, Operations, and Packaging Excellence (SCOPE) track this year at the 2021 ISPE Annual Meeting & Expo. Just like its name, this track will cover a wide...
Featured in this edition of iSpeak Reading Roundup, are the top blog posts from July 2021. Discover key insights for cleaning validation practices, risk-based approaches to quality, and more for what the pharmaceutical industry was reading last month.
COVID-19 has caused disruptions and delays with supply chain all across the world. Single-Use technologies (SUT) play a vital role in manufacturing of pandemic vaccines and have drastically reduced the typical vaccine development and manufacturing production timelines. The pandemic has further increased the sourcing demands for SUT. This non-forecasted demand has led to extended lead times or...
“This is the best time to be working in the life sciences industry,” the Keynote speaker said at an ISPE conference several years ago. At the time, I thought to myself, she is so right, this really is the best time to be working in the life sciences industry. Then, no one imagined that the world would be plunged into the worst of times battling a global pandemic that claimed the lives of over...
Featured in this edition of the Pharmaceutical Engineering® Online Reading Roundup are suggested summer reading ideas—articles on important topics from recent issues to revisit when you have some down time this summer—or to enjoy for the first time in case you missed them. Learn more about knowledge management implementation, zero carbon, MES, and more.
ISPE’s second virtual Global Pharmaceutical Regulatory Summit, held on 16 June 2021, brought together regulators from European Medicines Agency (EMA), Australian Therapeutic Goods Administration (TGA), US Food and Drug Administration, and Center for Drug Regulation and Research, FDA Philippines to share their insights on application of quality risk management. They discussed the positive...
The San Francisco Bay Area Chapter has won numerous ISPE Awards by virtue of their contributions to the pharmaceutical industry. They continue to have their finger on the pulse of the local industry and respond with innovative and educational programs. The Chapter’s Emerging Leaders Committee is especially active and plans monthly events while also helping new graduates become successful in...
The life sciences industry is experiencing a real estate gold rush. Already prosperous, pre-pandemic, life sciences construction surged dramatically when the COVID-19 vaccines rapidly expanded the need for administrative, research and development, and manufacturing spaces in the biotechnology field. This has led to a vast expansion in medical and biotech construction that has not abated, even...