Validation is creating an evidence trail to show that an action, method, or system leads to a consistent and reproducible result. Validation is the collection and evaluation of data from the process design stage through commercial production, which establishes scientific evidence that a process or components of a process can consistently deliver a quality product. Process validation involves a series of activities taking place over the lifecycle of the product and process.
Join the Conversation
As an ISPE member you can engage with 22 active CoPs, including new communities focused on Artificial Intelligence and Sustainability. Connect with experts and join the conversations: Become an ISPE member
ISPE members: Get more involved by volunteering.
Guidance Documents
Data Integrity (12)
+GAMP® (12)
+Lifecycle Management (1)
+Microbiological & Viral Contamination Control (1)
+Process Analytical Technology (1)
+Quality Assurance (2)
+Quality by Design (1)
+Validation (16)
+Community Discussions
Community Discussions
Jun 19, 2025
Quality
Lifecycle Management
Validation
May 22, 2025
Apr 24, 2025
Validation
Apr 16, 2025
Information Systems
Regulatory
Advanced Manufacturing
Artificial Intelligence
Mar 28, 2025
Information Systems
Regulatory
Advanced Manufacturing
Artificial Intelligence
Feb 03, 2025
Jan 27, 2025
Pharmaceutical Engineering Magazine Articles
Webinars
Upcoming
On-Demand
White Papers
March / April 2024
Navigating the Asia Pacific Pharmaceutical Landscape for Global Impact Cover: The Asia Pacific…
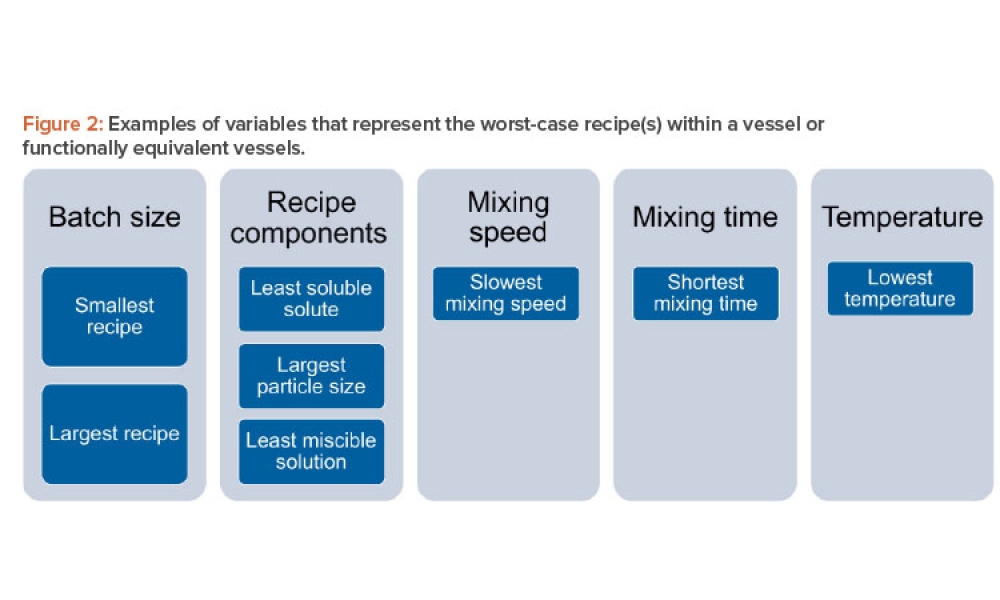
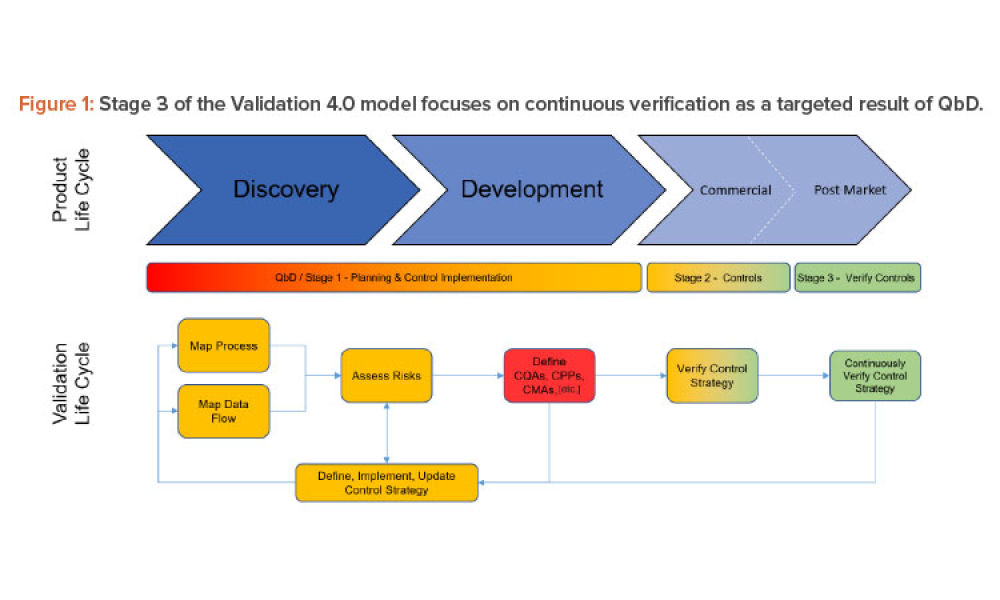
Stage 3 Process Validation: Applying Continued Process Verification Expectations
This discussion paper proposes ideas for answering the questions “How is Stage 3 monitoring and…

Pharmaceutical Job Board
Videos
iSpeak Blog Posts
Featured Conferences