Why is Good Engineering Practice important to the pharmaceutical industry?
Good engineering practice provides standardized approaches that provide a high degree of certainty that design and delivery of engineered systems are successful, safe, and cost-efficient. In short, good engineering practice is good business practice. However, beyond good business practice, for pharmaceutical engineers, good engineering practice exists in the context of the regulated industries and for the purpose of supporting and enabling Good Manufacturing Practice (GMP). The ultimate purpose of GMP is to provide benefit, and to prevent harm, to the end user (patient) of the drug product. Thus, by extension, part of the purpose of good engineering practice is to provide benefit, and to prevent harm, to the end user of the drug product produced with a process that uses engineered systems designed, delivered, and operating in a manner fit for purpose.
Ultimately, as with “good” regulated practice (GxP), good engineering practice is “good” as a result of reliable, cost-effective delivery of quality of work. With good manufacturing practice, manufacturing practices are “good” when they reliably result in manufacturing of drug products that meet their quality attributes, specifications, and requirements. Likewise, engineering practices are “good” when they reliably result in project deliverables that meet stakeholder requirements and engineered systems that are designed, delivered, and operate throughout their lifecycle, from conceptual design through decommissioning, in a manner fit for purpose and meeting user requirements and stakeholder expectations. Therefore, particularly for regulated industries, engineering practices are “good” when they result in manufacturing facilities and systems that function efficiently, safely, and reliably to produce a product that meets quality specifications. However, the motives for aspiring to practice “good engineering” are wider than the need to comply with GxP regulatory expectations; they encompass productivity, safety, and other business-related drivers.

What are the main updates or changes in the new edition?
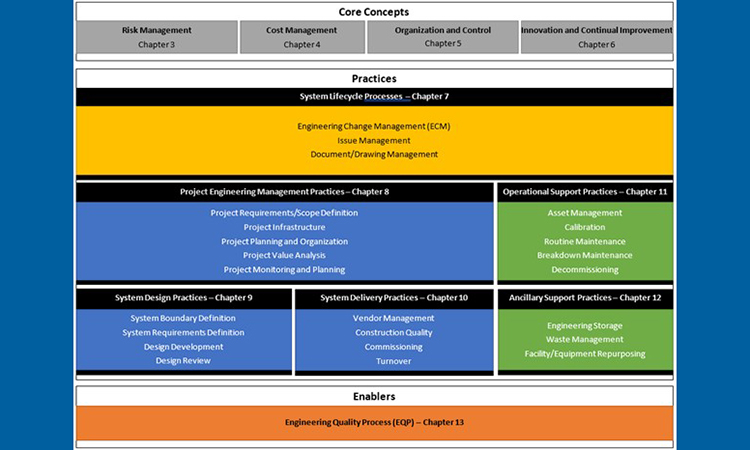
This revision of the Good Engineering Practice Guide considers the entire range of pharmaceutical engineering activity and identifies key attributes of good engineering practice within it, including how good engineering practice relates to and interfaces with GxP. The scope of good engineering practice covers the complete lifecycle of engineering projects and engineered systems from conceptual design to retirement. Good engineering practice provides a foundation required across the pharmaceutical industry that other areas, such as GxP, build upon.
This revision of the good engineering practice Guide, rather than merely listing the many processes and practices that fall within the scope of good engineering practice, organizes and visualizes good engineering practice as a holistic, lifecycle process for the design, delivery, and operation of engineered systems: