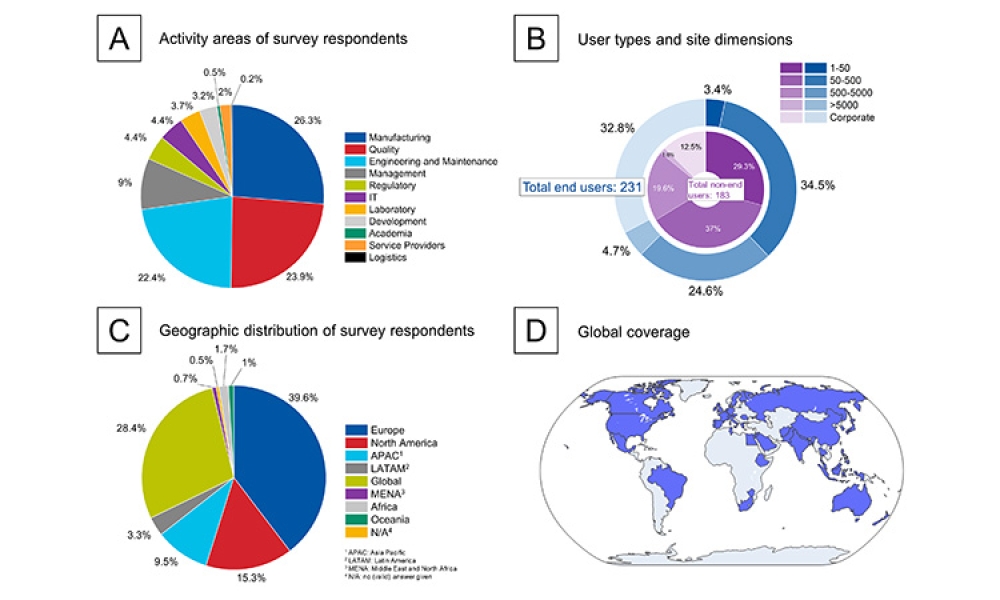
This article summarizes the key findings from the 7th Pharma 4.0™ Survey, conducted in 2023. It explores the demographics of the survey respondents, maturity levels of Pharma 4.0™ adoption, enabling technologies being leveraged, anticipated benefits, and challenges encountered.

Downloads
PIC/S in Latin America: Harmonization of cGMP Procedures
Cover: This article offers an overview of the benefits of Pharmaceutical Inspection Co-operation Scheme (PIC/S) membership for regulatory authorities and industry. It also highlights Latin American regulators’ current perspectives on PIC/S membership to increase awareness and encourage open dialogue about harmonization, recognition agreements, and potential increase for export facilitation, all which will help increase the access of high-quality medicines to patients. Given that only three countries are PIC/S members in the Latin America region, this represents a huge opportunity for cGMP inspection.
Global Collaborative Review: Understanding Overall Control Strategy and Patient Benefit-Risk
Feature: The pharmaceutical industry faces considerable challenges throughout the development, manufacturing, and supply of medicines, largely due to the intricate and divergent global regulatory landscape. The adoption of structured data standards and utilization of cloud-based platforms offer immense potential to overcome these challenges by facilitating faster and more efficient global collaborations between health authorities and industry. This potentially can be achieved by using a visual roadmap of the overall control strategy to help understand patient benefit-risk more effectively.
Industry Perspectives on Practical Application of Platform Analytical Procedures
Feature: Pharmaceutical and biotechnology companies employ platform analytical procedures in the development stages of their synthetic and biological drug products and are beginning to leverage them for commercial products. This shift is supported by the acceptance of platform procedures in the recently adopted ICH Q2(R2) and ICH Q14. Six case studies are shared in this article to highlight how platform procedures are developed, applied to products in development, and assessed for extent of validation needed to determine if appropriate for use.
FDA’s 2011 Process Validation Guidance: 10 Years On
Technical: In 2011, the US Food and Drug Administration (FDA) introduced the revised “Guidance for Industry: Process Validation: General Principles and Practices." The document incorporated principles from existing ICH guidance in place since 2005 (ICH Q8 and Q9) and 2008 (ICH Q10). ISPE formed their Product Quality Lifecycle Implementation (PQLI)® initiative to provide guidance on the practical implementation of the concepts described in these ICH guidelines.
Potency Measurements for Cellular and Gene Therapy Products
Technical: Cell and gene therapy (C>) products address various diseases at the cellular or genetic level, offer innovative treatment approaches, and represent a significant advancement in the field of medicine. However, developers of C> products face unique challenges due to their complexity, such as establishing assays that show a clear link between potency, mechanism of action (MoA), and clinical performance. Sponsors face a significant risk of a clinical hold if an adequate “potency assay” has not been established by the pivotal phase of clinical trials.
Finding the Assurance in Computer Software Assurance
Technical: Computer software assurance (CSA) has been discussed widely in industry over the past five years. While the principles are well understood and welcomed, until now some of the practical detail on how exactly to implement CSA into an organization has been missing.
In This Issue
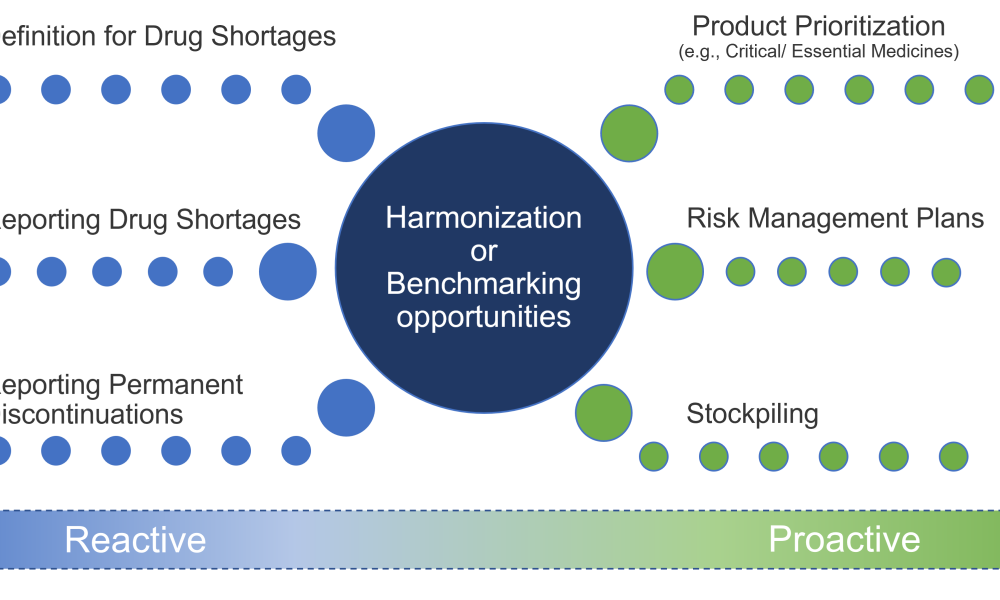
The regulation of drug shortage prevention has rapidly changed over the past several years due to large-scale, highly visible events, such as the COVID-19 pandemic, hurricanes, and geopolitical issues. Efforts to effectively address the complex and multifaceted issues contributing to drug shortages require close technical collaboration and clear communication between the pharmaceutical...
After completing her doctoral degree at Oklahoma State University, Sarah Pope Miksinski received a fellowship at the National Institutes of Health. While working there, she realized that research was not the best career path for her.
The pharmaceutical landscape is rapidly evolving, and cell and gene therapies (C>) are at the forefront of this transformation. These therapies are revolutionizing how we approach patient care, particularly in the realm of personalized medicine. However, this innovation has also introduced challenges, especially when establishing new manufacturing facilities.
Drug delivery devices have become an essential component for many modern medical therapies, and it’s vital that they function as intended. However, the reality of marketed products shows that this is not always achieved because drug-device combination products are becoming increasingly complex, with an increasing number of potential failure modes. Significant challenges for engineers include...
Computer software assurance (CSA) has been discussed widely in industry over the past five years. While the principles are well understood and welcomed, until now some of the practical detail on how exactly to implement CSA into an organization has been missing.
Cell and gene therapy (C>) products address various diseases at the cellular or genetic level, offer innovative treatment approaches, and represent a significant advancement in the field of medicine. However, developers of C> products face unique challenges due to their complexity, such as establishing assays that show a clear link between potency, mechanism of action (MoA), and...
In 2011, the US Food and Drug Administration (FDA) introduced the revised “Guidance for Industry: Process Validation: General Principles and Practices.”
Anil Mathai first heard about ISPE 30 years ago. “I attended Drexel University, where you are required to complete three cooperative education jobs. One of mine was for Rhône-Poulenc Rorer, Inc. in Collegeville, Pennsylvania. While I was there, I learned about validation and decided that I wanted to be in pharmaceuticals as a chemical engineer.”
In each issue of Pharmaceutical Engineering®, we introduce a member of the ISPE staff who provides ISPE members with key information and services. Meet Wendy McGhee, Health Authority Outreach Manager in the Regulatory Operations group.
Pharmaceutical and biotechnology companies employ platform analytical procedures in the development stages of their synthetic and biological drug products and are beginning to leverage them for commercial products. This shift is supported by the acceptance of platform procedures in the recently adopted ICH Q2(R2) and ICH Q14. Six case studies are shared in this article to highlight how...
The pharmaceutical industry faces considerable challenges throughout the development, manufacturing, and supply of medicines, largely due to the intricate and divergent global regulatory landscape. The adoption of structured data standards and utilization of cloud-based platforms offer immense potential to overcome these challenges by facilitating faster and more efficient global...
In 2023, ISPE launched an expansive and significant initiative, Enabling Global Pharmaceutical Innovation: Delivering for Patients, to address the barriers to technological innovation in the pharmaceutical industry. The first activity of the initiative was to conduct a three-part survey of ISPE members to understand the circumstances and confirm the sources that create barriers to...
ISPE’s regulatory initiatives and programs bring visibility and solutions to significant regulatory and quality challenges faced by the industry, facilitate the flow of information between ISPE members and global health authorities to find solutions, and promote interaction between regulatory bodies in the interests of harmonization.
We are excited about the 2024 ISPE Annual Meeting & Expo in Orlando, Florida! A special thank you to the sponsors and planning committee for the Hackathon. We appreciate your commitment to ensuring that recent graduates and college students will benefit from this challenging and...
In the dynamic realm of regulatory affairs, a significant transformation is unfolding—a movement that empowers women to assume pivotal roles in decision-making, policy formulation, and leadership.
I hope everyone had the opportunity to enjoy the summer. The ISPE International Board of Directors and ISPE staff have kept busy through the summer months, launching two new Communities of Practice: Sustainability and Artificial Intelligence.
On 13 March 2024, ISPE concluded the 2024 Aseptic Conference with a regulatory panel question and answer session. Attendees were invited to submit questions to representatives from the Austrian Agency for Health and Food Safety (AGES), US Food and Drug Administration (FDA), Regierungspraesidium Tübingen (RP Tübingen), World Health Organization (WHO), Therapeutic Goods Administration (TGA), and...
This article offers an overview of the benefits of Pharmaceutical Inspection Co-operation Scheme (PIC/S) membership for regulatory authorities and industry. It also highlights Latin American regulators’ current perspectives on PIC/S membership to increase awareness and encourage open dialogue about harmonization, recognition agreements, and potential increase for export facilitation, all which...