The 2022 ISPE Aseptic Conference Regulatory Panel session on 15 March was as always one of the highlights of the conference, and this year’s discussion with regulators followed in that tradition.

Downloads
Is a Globally Harmonized Quality Overall Summary Possible?
Cover: The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) guideline on Registration of Pharmaceuticals for Human Use (M4) offers advantages in the consistent format of the registration dossier using the Common Technical Document (CTD). However, it does not deliver a comprehensive view of the overall manufacturing control strategy or a means of understanding and managing the quality of the product throughout its life cycle. As a result, several regulatory authorities that have implemented the CTD format have also insisted on supplementary quality summary documentation that exceeds ICH requirements, and, in effect, creates divergent expectations for chemistry, manufacturing, and controls (CMC) content. A single global quality overall summary (QOS) format could clearly convey a holistic view of a product’s control strategy and improve the efficiency and economy of the regulatory review of an application while providing a way for the applicant and reviewer to align on a product life-cycle management plan.
Streamlining Postapproval Submissions Using ICH Q12 & SCDM
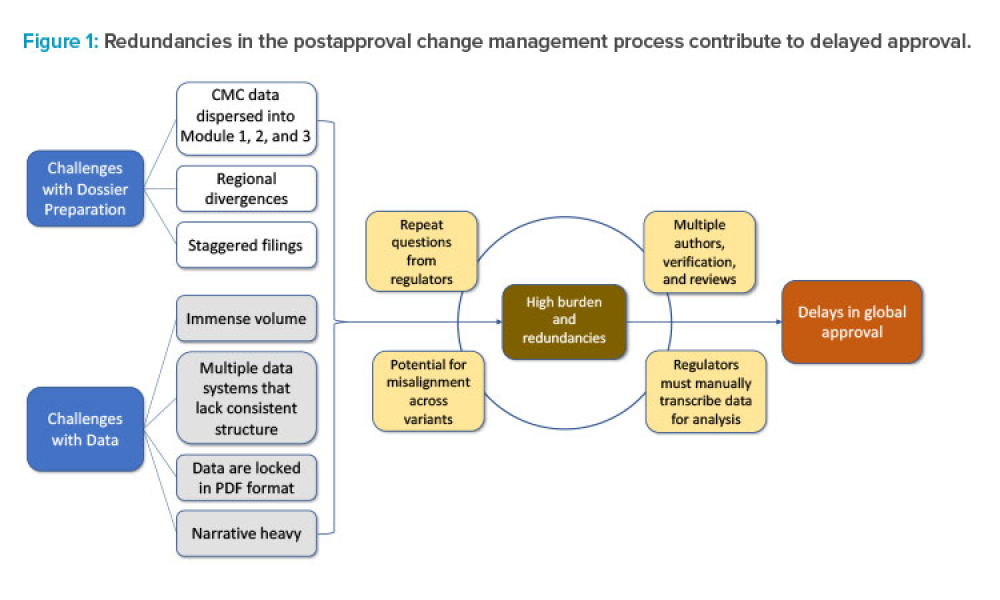
Feature: Postapproval change management of pharmaceuticals is an essential part of life-cycle management but is associated with regulatory challenges. Incorporating concepts and tools from the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) Q12 guideline, combined with structured content and data management (SCDM) and a cloud-based data exchange platform, could provide synergistic benefits that will enable efficient supply maintenance of life-saving therapies worldwide.
Regulatory Landscape for Raw Materials: CMC Considerations
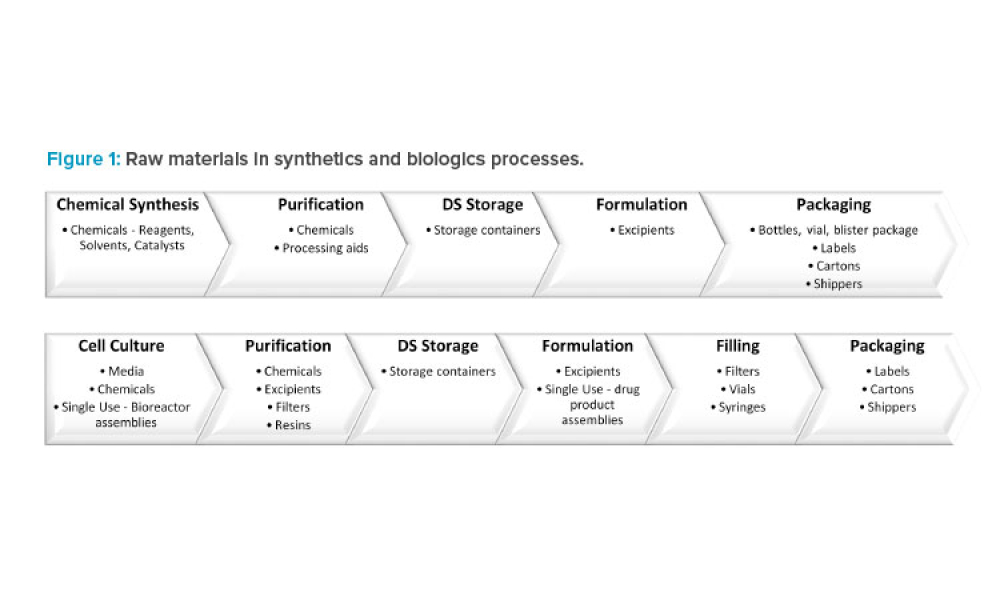
Feature: A reliable supply of raw materials is critical to maintain a robust supply chain to serve patients globally. With shortages, regulatory complexity is compounded due to differences in submission and data requirements from various regulatory agencies. Therefore, there is an increasing need to implement a harmonized regulatory infrastructure that is both flexible and predictable to provide more agility without product delays.
Validation 4.0: Case Studies for Oral Solid Dose Manufacturing
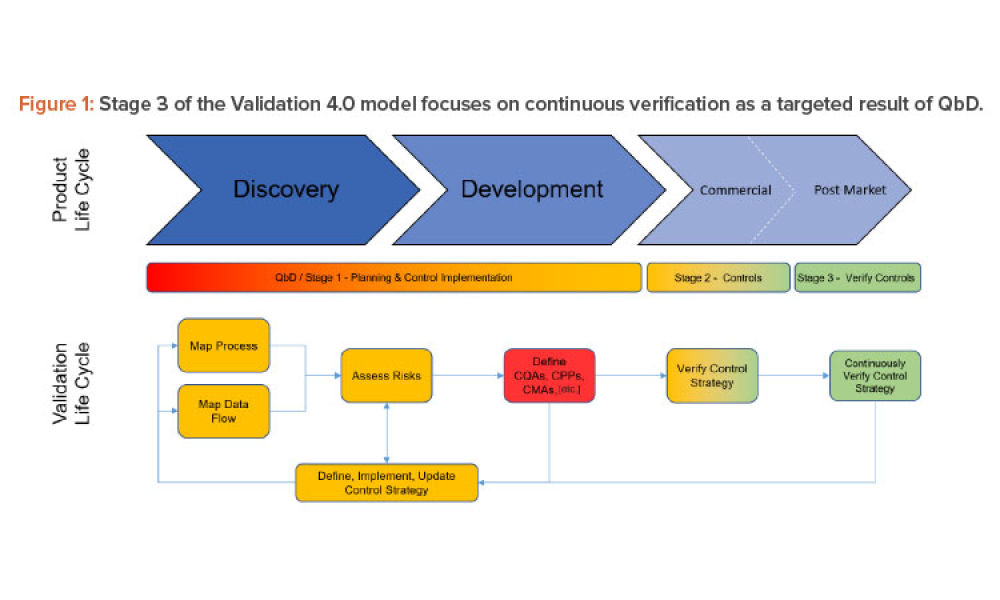
Technical: Three case studies on Validation 4.0 demonstrate how quality by design (QbD) principles, when applied with digitization, can verify processes in scale-up and technology transfer, and why blend and content uniformity matter for tablet integrity.
Novel Dry Decontamination Method Using: Gaseous Chlorine Dioxide
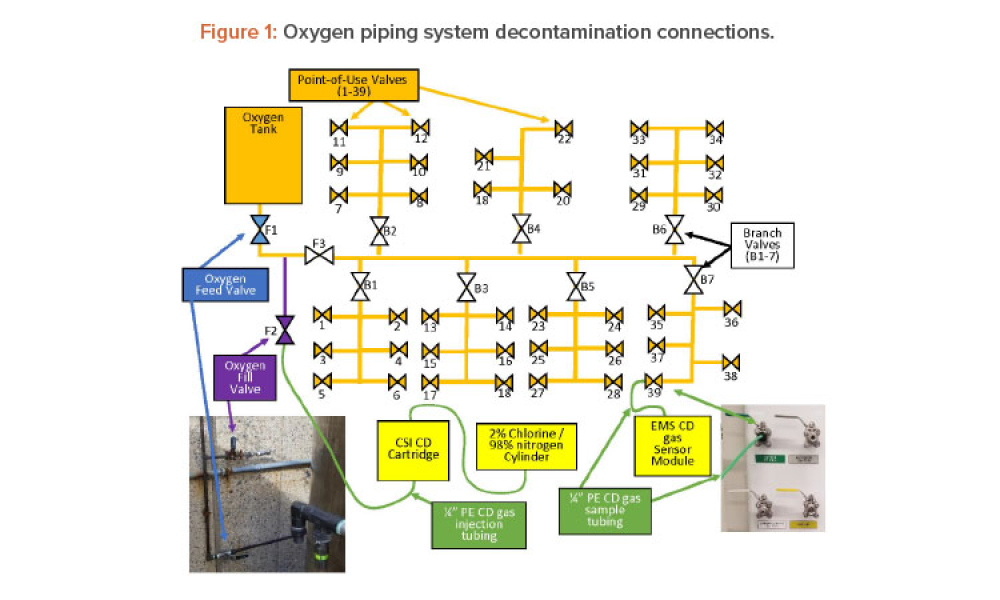
Technical: Chlorine dioxide has been shown effective in decontaminating various types of chambers and volumes such as rooms, isolators, processing tanks, and entire facilities, but its use to decontaminate compressed gas piping systems has not been documented. This article discusses using dry gaseous chlorine dioxide (ClO2) to decontaminate an oxygen (O2) feed piping system in a pharmaceutical research laboratory and shows that a dry gas can be used to remediate a contaminated piping system.
In This Issue
Postapproval change management of pharmaceuticals is an essential part of life-cycle management but is associated with regulatory challenges. Incorporating concepts and tools from the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) Q12 guideline, combined with structured content and data management (SCDM) and a cloud-based data exchange...
A reliable supply of raw materials is critical to maintain a robust supply chain to serve patients globally. With shortages, regulatory complexity is compounded due to differences in submission and data requirements from various regulatory agencies. Therefore, there is an increasing need to implement a harmonized regulatory infrastructure that is both flexible and predictable to provide more...
Three case studies on Validation 4.0 demonstrate how quality by design (QbD) principles, when applied with digitization, can verify processes in scale-up and technology transfer, and why blend and content uniformity matter for tablet integrity.
Chlorine dioxide has been shown effective in decontaminating various types of chambers and volumes such as rooms, isolators, processing tanks, and entire facilities, but its use to decontaminate compressed gas piping systems has not been documented. This article discusses using dry gaseous chlorine dioxide (ClO2) to decontaminate an oxygen (O2) feed piping system in a...
Summer is over and we are all back to our workplaces. This year has been different than the two previous years with vaccination against SARS-CoV 2 decoupling the infection rates from hospitalization, intensive-care units, and fatalities. That is an unprecedented achievement in such short time, and we as the pharmaceutical industry have helped make it happen to get back to a new normal.
Over the last two years, ISPE’s Women in Pharma® (WIP) initiative remained undeterred in its mission to connect women throughout the global pharmaceutical industry. Through virtual engagement opportunities—including book clubs,...
Most people in their careers have to work with others at some point, and in these work interactions there are exchanges of ideas. In pushing business processes forward, determining methodology for a new experiment, or designing a manufacturing facility, multiple stakeholders come from different points of view and with varying priorities. Working with others in any capacity and having the exact...
The members of the Sterile Products Processing (SPP) Community of Practice (CoP) Steering Committee are as diverse and varied as the topics they discuss. The Community of Practice is comprised of professionals from around...
The Europe Hackathon for students and recent graduates was held 23–24 April as part of the activities during the 2022 ISPE Europe Annual Conference. This article provides an overview of the event and how it...
Four teams of Emerging Leaders (ELs) represented one of four different company manufacturer types in the Emerging Leader Hackathon at the ISPE 2022 Europe Conference. This article provides some participants’ views of...
ISPE has more than 19,000 members from more than 129 countries, and many of them spend countless hours sharing their knowledge and connecting with others, helping ISPE to advance the educational and technical efficiency of all members.
The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) guideline on Registration of Pharmaceuticals for Human Use (M4)