ISPE starts 2022 with a bang, with the 31st Annual Face-to-Face 2022 ISPE Aseptic Conference, March 14-15, offering attendees several unique perspectives and case studies across various...
Submit Your Best Content to ISPE
ISPE’s official blog, iSpeak accepts contributions from our Members and professionals in the pharma industry.
Competitive advantage and sustained business results depend on leaders building and maintaining a strong culture. This has been reinforced by the increased challenges in attracting and retaining talent. The FDA is also placing significant focus on Quality Management Maturity. But how do leaders know if they are on track? Assessing leadership systems is often overlooked or much more difficult...
Vaccine Manufacturing
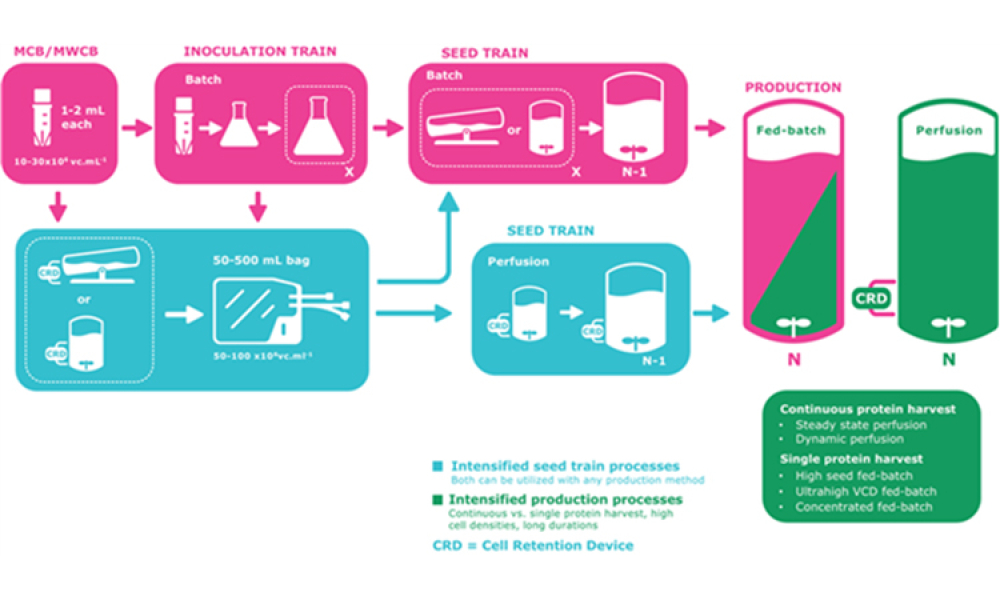
The industry is experiencing an acceleration in the development and approval of novel prophylactic and therapeutic vaccine modalities. Concurrently, many biopharmaceuticals are experiencing the benefits of manufacturing process intensification (PI). Describing such advances for cell-culture based processes is challenging in the field of vaccinology because of...
It is a great honour and pleasure for me as Chair of the ISPE Board of Directors and Chair of the 2022 ISPE Aseptic Conference Program Committee to give you an overview of the upcoming conference including information about the knowledge that will be shared, a look at some of the speakers, and details about a few of the networking opportunities.
Volunteers are the lifeblood of ISPE. Along with staff, they implement the global mission of the Society and produce essential content that benefits the life sciences industry. ISPE values our volunteers’ time and the commitment they make to sharing their knowledge and talents for the good of others. There are numerous ways to get involved with ISPE from serving on a committee to reviewing a...
In September 2021, a panel of regulators representing ANSM (France), ANVISA (Brazil), FDA (US), and the WHO participated in a webinar to discuss opportunities for drug shortage prevention. The discussion was facilitated by industry leaders while industry insights were obtained through polling of the global audience.
Expectations have never been higher for the pharmaceutical industry.
The uncertainty that comes with the introduction of a new idea has affected society for centuries. We all grew up hearing about how sailors of old feared sailing off the edge of the world before we accepted that the Earth was indeed round. Inventors are always ridiculed by naysayers until their ideas are adopted and become common place. It’s safe to say that most of us approach new ideas with...
Featured in this edition of iSpeak Reading Roundup, are the top blog posts from October 2021. Discover key insights for cleaning validation practices, risk-based approaches to quality, and more for what the pharmaceutical industry was reading last month.
The ISPE Aseptic Conference is in its 31st year. This year like many others, we have a long list of enigmatic speakers discussing topics that matter to your everyday business. But unlike...
Late last month, ISPE published the 2nd Edition of the ISPE Good Practice Guide: Good Engineering Practice. This revision comes a decade and a half after the original guide was...
Featured in this edition of the Pharmaceutical Engineering® Online Reading Roundup are popular articles that visitors to the site were reading during October. Revisit articles on cleanliness classifications, steam sterilization, pharma water systems, and more.
ISPE’s Facility of the Year Awards (FOYA) celebrates cutting-edge engineering, new technology, and unique collaborations. It provides a platform for the pharmaceutical science and manufacturing industry to showcase...
Over the past twenty years, an evolution of pharmaceutical development has resulted in new approaches to ensuring product quality and patient safety. The science and risk-based concepts outlined in ICH Q8-Q12 encourage a more holistic approach to the product control strategy. Under this paradigm, product quality should not be ensured by the quality specification, rather it should be confirmed....
Since the FDA issued final guidance on Data Integrity in 2018, the challenges have been many. The growth in observations has been substantial. With IT transforming dramatically over the last...
The final session of the 2021 ISPE Annual Meeting & Expo was the Global Regulatory Town Hall. It brought together regulators from around the world to provide insights about current issues including harmonization and convergence. This year’s panel session was heavily flavored by inputs about lessons learned by regulators during the pandemic, and what may change (or not) after pandemic.
The Membership and Awards Breakfast at the 2021 ISPE Annual Meeting & Expo on 2 November saw the traditional passing of the gavel in person for the first time in two years as Jörg...
The theme of the 2021 Annual Meeting & Expo is agility, collaboration, and innovation, and these were demonstrated from a number of perspectives in the keynote presentations during the...