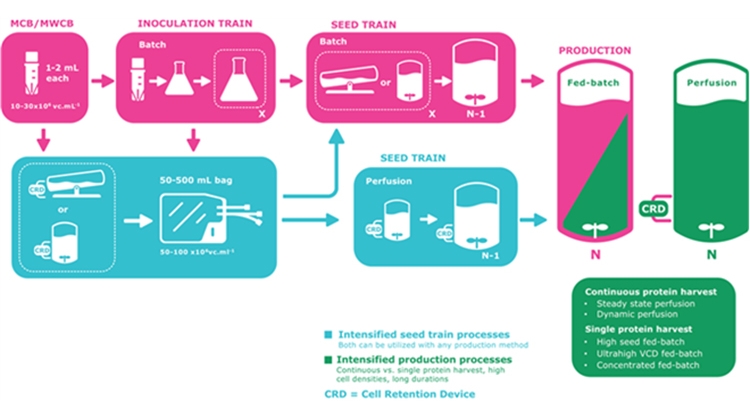
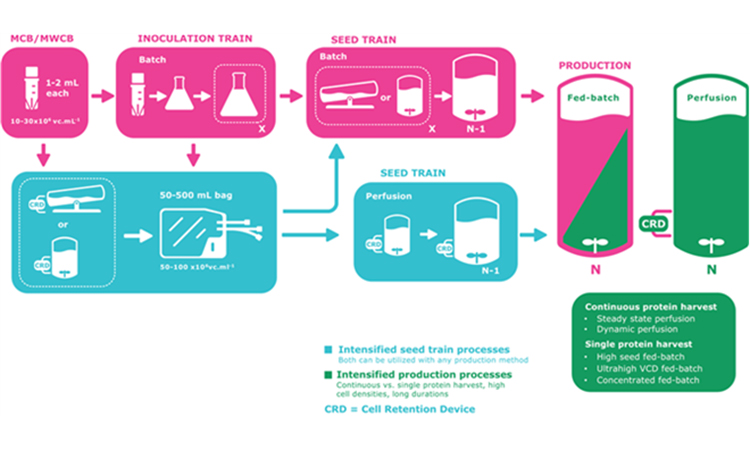
While promoted for many other values, various approaches to continuous bioprocessing can also dramatically increase productivity in terms of time, volume, and footprint. Modifying media composition, operating cell density, perfusion rate and culture duration in existing perfusion modes can provide further improvements.
PI through selection of the most appropriate bioreactor, process mode and control system is becoming more challenging. There were previously only a few, rather intuitive approaches − but even in examining stirred-tank suspension culture approaches, there are now many configurations to choose from. And other bioreactor types, such as fixed bed and hollow fiber do present even more options
After a decade of success in other industries, we now see “digital” approaches in vaccine manufacturing taking hold. This industry has recently seen such early “Pharma 4.0” initiatives as autonomous warehouses, paperless data recording, and electronic batch records. New data sources, as well as their means of transmission, storage, and curation are maturing rapidly. Current PI-related digital tools in vaccine manufacturing includes connectivity through industrial internet of things (IIoT), and eventually through the internet of everything (IoE).
Supervisory control and more powerful data acquisition are supporting model-based adaptive control systems addressing many variables and actuating dynamic changes in new output variables. New artificial intelligence (AI) applications are further empowering PAT and QbD in harnessing the massive amounts of process data generated by new probes, sampling technologies, monitoring instrumentation, as well as automated high-throughput and multiplexed analytics.
“Digital twins” are in-silico model providing a digital transformation of each operation− supporting, analysis, prediction, control, and optimization of the manufacturing process. AI and machine learning will soon interface with them to provide novel solutions to complex problems, even with less than optimally governed and labeled data. It will make appropriate decisions for bioprocess development, predictions, recommendations, and control using advanced monitoring, big data processing capabilities and new industrial connectivity.
The speed and power of data processing hardware, as well as the capability of new optical, chemical, and physical sensors continue to grow. Improvements in the monitoring of existing parameters, and well as entirely new ones are being enables by such powerful developments as capillary electrophoresis integrated mass spectrometry,, , , monolith immobilized enzymes providing on-line digestion of proteins, and smart sensors converting surrogate measurements to functional values in silico.
In process development activities for intensification, there is growth in the use of automated parallel microbioreactor systems capable of running as either high inoculation fed batch and or as perfusion mimics. Developments in clone selection techniques include new criteria, such as culture behavior and robustness in the actual production media and mode, and the early use of product quality profiling. Cloning, cell-line development and gene editing are being advanced greatly through new applications of microfluidics.
Finally, qualified vendors of facility design and build now specifically support many of the intensification initiatives mentioned above, integrating laboratory information management systems (LIMS) with enterprise resource planning and control. Orchestration of such digital technologies as cloud- and edge-based systems, process automation, and AI supports advanced production layouts. This, as well as features of modular and prefabricated design, enable both process flexibility and intensification in a data-intensive landscape.