Annex 1 of the EC GMP Guide "Manufacture of Sterile Medicinal Products" has a long history. First published in 1989, there have been a total of 5 adaptations in 1996, 2003, 2005, 2007 and 2009, but no complete revision. In 2012, there was a proposal for a complete revision which resulted in a concept paper in 2015. The first draft for comments from industry stakeholders was...
Submit Your Best Content to ISPE
ISPE’s official blog, iSpeak accepts contributions from our Members and professionals in the pharma industry.
It may seem that the only things loved by pharma industry more than acronyms are new buzz phrases. And that may be true. But sometimes, behind the buzz phrase, there are real, strong, achievable benefits to our work, our industry, and our end patients.
Single-use plastics are critical for healthcare, life sciences laboratories, and bioprocessing. They have many benefits over other materials and their use is increasing. In particular, single-use technologies for bioprocessing decrease costs, reduce water and energy usage, and increase efficiency and scalability. However, most of these high-value, virgin materials end up in landfills and...
The industry is continuously evolving to meet new requirements. Many of these requirements include adhering to global regulatory harmonization, improving supply chain robustness, minimizing drug shortages, reducing the complexity of managing product life cycles, and reducing the environmental impact of pharmaceutical processes. At the
Winners in this category exemplify the novel application of process manufacturing techniques, innovative design concepts, new technologies and unique solutions that exemplify the next generation of agile, flexible, efficient and effective new and existing pharmaceutical and biotechnology facilities. This includes implementation of commercially available and custom developed equipment which...
Winners in this category exemplify application of novel approaches, standards, and practices which result in efficient processing, resourceful utilities, and business advantage by increasing patient access and preventing drug shortages through in-country-for country manufacturing; outbreak, epidemic, or emerging health crisis response via rapid deployment and fast-track drug production; and...
Do you have your finger on the pulse of regulatory concerns in the Asia-Pacific Region? Would you like to discuss strategies to meet regional challenges? Are you interested in regulatory harmonization? If so, we’d like to re-introduce ISPE’s Regulatory Quality Harmonization Committee (RQHC)’s Asia-Pacific Regional Focus Group (A-P RFG).
At the 2022 ISPE Europe Annual Meeting held in Madrid on 25-27 April 2022, Evdokia Korakianiti, Head of Quality and Safety, European Medicines Agency (EMA), gave a keynote presentation of EMA’s approach to...
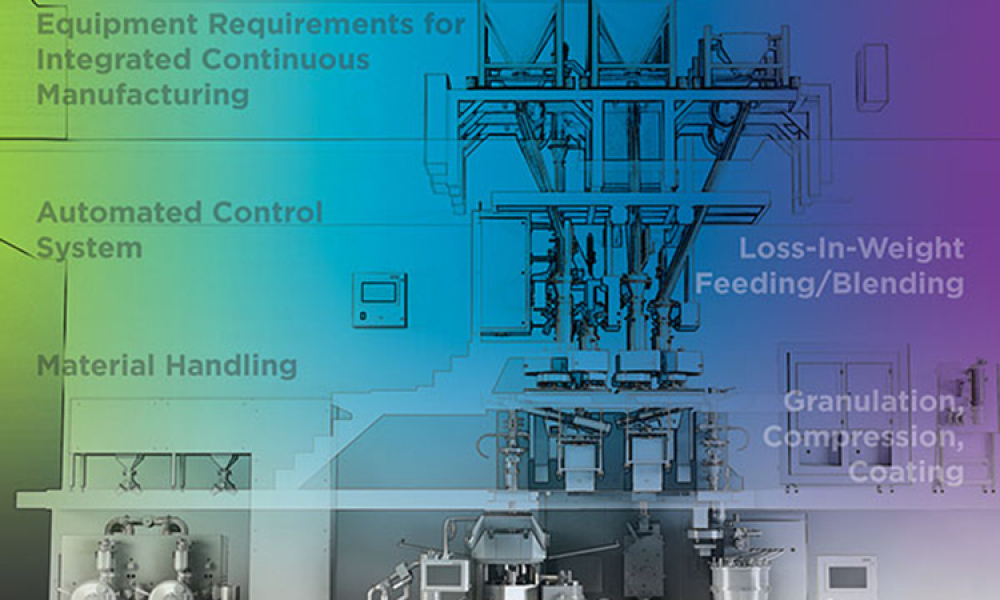
As the benefits of continuous manufacturing became more apparent in the pharmaceutical industry, companies looked for ways to incorporate the technology into their manufacturing processes. In 2017 The ISPE Oral Solid Dosage (OSD) Community of Practice (CoP) formed a working team to advance the use of continuous manufacturing in the pharmaceutical industry and to increase the long-term...
Are you considering joining ISPE? Or maybe you’ve recently joined the society or are a longtime member looking to better understand all the benefits available to you. If so, you’ve come to the right place!
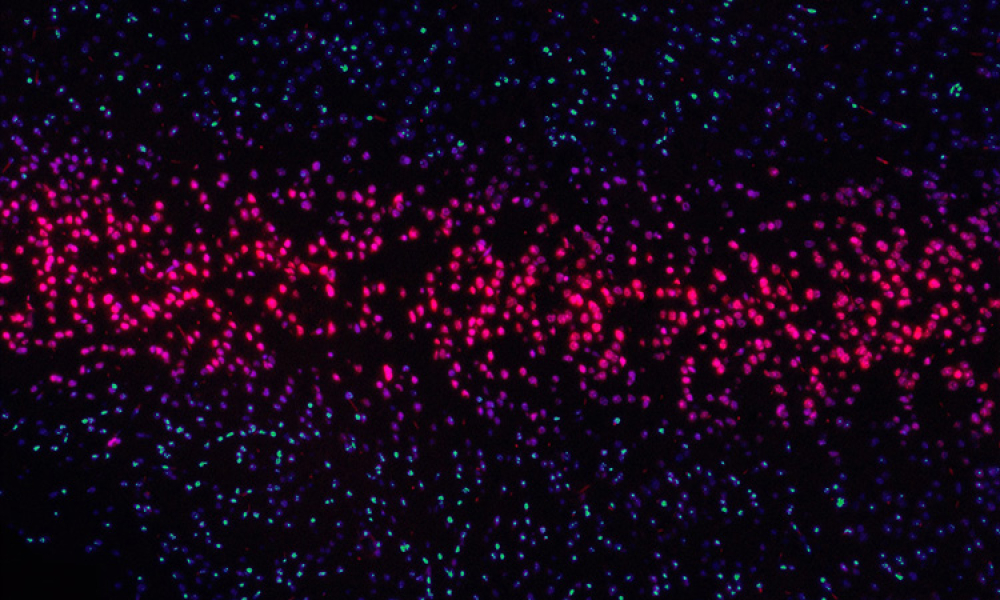
ATMPs are based on genes, cells or tissues delivered to patients to provide a therapeutic benefit based on a specific target of interest. Often referred to as ‘Personalised Medicine’. A sector of healthcare that is rapidly evolving and expanding with some unique challenges such as microbial contamination and product variability. Traditional manufacturing processes are for synthetically derived...
GAMP® 5 Second Edition is here! Since its publication, GAMP® 5 has been far and away the leading international guidance on GxP computerized systems validation and compliance, and it was time to update our guidance to...
That question was at the heart of the of ISPE’s “Expert Xchange: Regulatory Summit on ICH Q9 Revision” held 9 June 2022. Seventy-one participants from 14 countries discussed ICH Q9(R1) and received valuable insight from several members of the Expert Working Group (EWG) assembled in 2020 to lead the ICH Q9 revision process. Discussion centered on the current state of application of quality risk...
At the ISPE Europe Annual Meeting held in Madrid on 26 April 2022, Nandini Rakala, Data Scientist & Visiting Associate, Office of Pharmaceutical Quality, CDER, FDA gave a keynote presentation on FDA’s...
Winners in this category exemplify the application of novel tools and approaches to delivering projects that improved efficiencies, overcame unusual challenges, promoted effectiveness, and organized stakeholders and project team participants in ways that led to successful outcomes such as efficiency, delivery, quality, product yield, consistency, and cost of goods.
Featured in this edition of the Pharmaceutical Engineering® Online Reading Roundup are some favorites for your summer reading pleasure on a topic that is always important: supply chain issues.
As the world’s second largest market for pharmaceuticals and accounting for 20% of global medical device sales with double digit annual growth, the Chinese market is poised to have a significant impact on expanding patient access to innovative therapies and combination products (CPs).
Digital transformation is the novel use of digital technology, including instrumentation and control systems, to solve traditional problems in a transformative manner. These digital solutions enable inherently new types of innovation and creativity, rather than simply enhancing, and supporting traditional methods.