Dave DiProspero, Director of Pharmaceutical Process Technology, CRB, and Gregory Connelly, PhD, Senior Director – Continuous Manufacturing, Vertex Pharmaceuticals are members of that team and co-leads of the recently published ISPE Good Practice Guide: Continuous Manufacturing of Oral Solid Dosage Forms.
Dave DiProspero, Director of Pharmaceutical Process Technology, CRB, and Gregory Connelly, PhD, Senior Director – Continuous Manufacturing, Vertex Pharmaceuticals are members of that team and co-leads of the recently published ISPE Good Practice Guide: Continuous Manufacturing of Oral Solid Dosage Forms.
What are the benefits of continuous manufacturing and why is this technology important in the pharmaceutical industry?
Gregory Connelly: The general consensus among experienced practitioners involved in Oral Solid Dose Form Manufacturing is that the continuous manufacturing approach has numerous benefits over the traditional batch process. It is typically more robust and designed for reduced variability, and it can achieve improved product quality through better blend uniformity. Continuous manufacturing provides for a full range of the product life cycle, from small volume clinical production to large volume commercial production, with minimization or elimination of scale up activities, all leading to Real Time Release.
Dave DiProspero: In addition, continuous manufacturing offers potential safety benefits such as contained operations and lower exposure risk for operating personnel. Add to this the significantly smaller facility footprint required for a continuous manufacturing operation and both Cap-x and Op-x costs are greatly reduced.
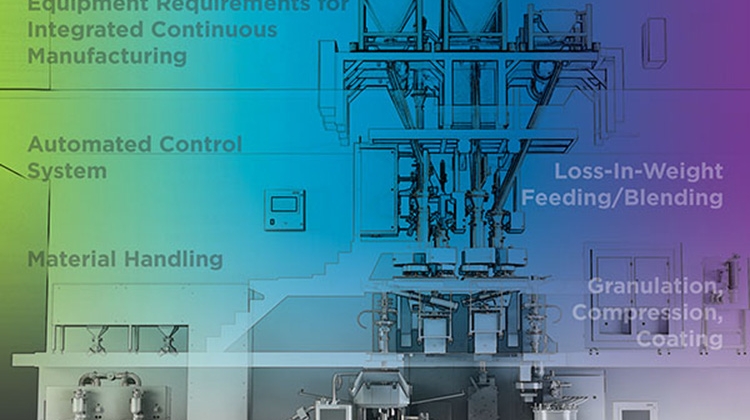
How could the ISPE Good Practice Guide: Continuous Manufacturing of Oral Solid Dosage Forms help companies who are hoping to transition production from batch manufacturing to continuous manufacturing?
Dave DiProspero: Over the past decade, the pharmaceutical industry has been applying the principles of continuous processing to the manufacture of oral solid dosage medicines. International Regulatory Agencies, following the lead of the FDA, have provide encouragement and endorsement of continuous manufacturing and the technology has greatly advanced.
While the industry initially focused on establishing the technical capabilities of continuous manufacturing systems, it has now advanced to the commercial manufacture of multiple drug products with distribution approved in the United States, Europe, and other countries around the globe and includes many more in development.
Gregory Connelly: This guide is intended to serve as a comprehensive reference, providing guidance for pharmaceutical companies, regulators, engineering firms and vendors engaged in this emerging technology. This guide provides a comprehensive review of Continuous OSD Manufacturing and was produced with the following objectives in mind:
- To aid in the general understanding of Continuous OSD Manufacturing
- To establish a set of minimum equipment requirements for various pharmaceutical unit operations to be integrated as components of a CM line, including design considerations for the required automated controls
- To identify opportunities for future harmonization and flexible integration of both physical equipment and control software
- To identify areas where currently available equipment can be enhanced to meet the needs of the industry as the next generation of CM platforms are developed.
Purchase Guide
Join the Oral Solid Dosage Community of Practice