PIC/S has more than 50 member countries and is actively collaborating with the EMA and WHO on the development of the revised EudraLex Annex 1 for Sterile Medicinal Products. PIC/S is considered to have the most harmonised set of guidelines for the industry and therefore ideally positioned to influence the development of future regulations and guidelines. However, PIC/S has started to deviate from the EMA with respect to the guidance for ATMPs. The EMA has removed all reference to ATMPs in their Annex 2 regulations and instead created a new standalone set of regulations specific to ATMPs in the form of EudraLex Volume 4, Part IV. PIC/S has decided to keep the ATMP guidance in Annex 2, but create a separate Annex 2A for ATMPs with Annex 2B specific to biological medicinal substances and products for human use as per EudraLex Annex 2. PIC/S Annex 2A is very different to the EMA regulations in that all the other applicable Annexes contained within PIC/S, such as Annex 1 for the Manufacture of Sterile Medicinal Products, still apply to ATMPs. Unless specifically referenced in EudraLex Part IV, this is not the case.
The EMA regulations and PIC/S guidance for ATMPs were published on the following dates:
| ATMP Regulation / Guidance | Date |
|---|
| EudraLex Volume 4 Part IV Guidelines on Good Manufacturing Practice specific to Advanced Therapy Medicinal Products | 22 November 2017 Enforcement date 22 May 2018 (88 Pages) |
| PIC/S Annex 2A Manufacture of Advanced Therapy Medicinal Products for Human Use | 1 February 2022 (49 Pages – almost half the number of pages) |
The new guidance for ATMPs released by PIC/S in some cases contains different information to the corresponding EMA regulations. It is important to highlight those differences so that they may be taken into consideration when designing and operating ATMP facilities.
PIC/S Annex 2A does not contain a section dedicated to risk-based approaches although it does refer to QRM throughout the guideline similar to the new and anticipated revision of EudraLex Annex 1. EudraLex Part IV contains a lot of information on risk based approaches including the possibility of manufacturing in a critical Grade A area with a Grade C background (EudraLex Part IV, Section 2.50 on Page 14), i.e. when performing very early phase / proof of concept trials for a life threatening condition when there are no alternative treatments available. An option that is not referred to in PIC/S Annex 2A.
It is interesting that PIC/S Annex 2A refers to a Contamination Control Strategy (CCS). A new concept which will be introduced in the revised version of EudraLex Annex 1. The close collaboration between the EMA and PIC/S during the development of the revised EudraLex Annex 1 is evident from PIC/S Annex 2A.
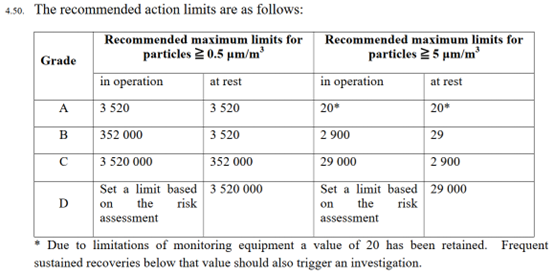
EudraLex Part IV refers to the need to set a limit for Grade D non-viable ‘in-operation’ particulate monitoring based on a risk assessment (EudraLex Part IV, Section 4.50, Page 25). EudraLex Annex 1, PIC/S Annex 1 and Annex 2A do not require particulate monitoring for Grade D ‘in operation’, but the revised EudraLex Annex 1 version does contain this requirement. See table below from EudraLex Part IV for ATMPs.
In summary, there are no fundamental differences between PIC/S Annex 2A and EudraLex Part IV. However, there are some differences that are worth highlighting. For example, there is more clarity in PIC/S Annex 2A regarding the types of ATMPs that are included in scope (illustrated in Table 1 and Figures 1 to 3 of Annex 2A). The same information does reside in a Q&A publication by the EMA (EMA/246400/2021, dated 24 February, 2021), but is not referenced in EudraLex Part IV and therefore not easily located. PIC/S Annex 2A is aligned with the anticipated revision of EudraLex Annex 1 (Sterile Medicinal Products) by referencing ‘Quality Risk Management’ and a ‘Contamination Control Strategy’. Although not necessarily excluded, PIC/S Annex 2A does not refer to the possibility of manufacturing in a critical Grade A area with a Grade C background for very early proof of concept trials for a life threatening condition when no alternative treatment exists. Finally, as per the anticipated EudraLex Annex 1 revision, EudraLex Part IV refers to the need to perform a risk assessment to determine if Grade D non-viable ‘in-operation’ particulate monitoring is required; a requirement that is currently not included in PIC/S Annex 1 or Annex 2A.
| | PIC/S | EudraLex, Volume 4 (Europe) |
|---|
| GMP Requirement / Approach | Annex 2A | Part IV | Annex 1 (Revised) |
|---|
| Quality Risk Management | X | | X |
| Contamination Control Strategy | X | | X |
| Risk Assess Grade D ‘In Operation’ for Non-Viable Particulate Monitoring | | X | X |
| *Standalone Regulations / Guidance | | X | |
*Note PIC/S Annex 2A is connected with PIC/S Annex 1 which is not the case with EudraLex, Volume 4, Part IV unless specifically referenced in the regulations. EudraLex, Volume 4, Part IV may therefore require additional alignment when the equivalent EudraLex Annex 1 is revised. It may also explain why EudraLex, Volume 4, Part IV is almost double the number of pages when compared to PIC/S Annex 2A.
Disclaimer:
This is a brief and informal synopsis of a discussion among regulators from various countries and regulatory organizations held during the “Expert Xchange: Regulatory Summit on ICH Q9 Revision” on June 9, 2022. It has not been vetted by any of the agencies or regulators cited in this article.






