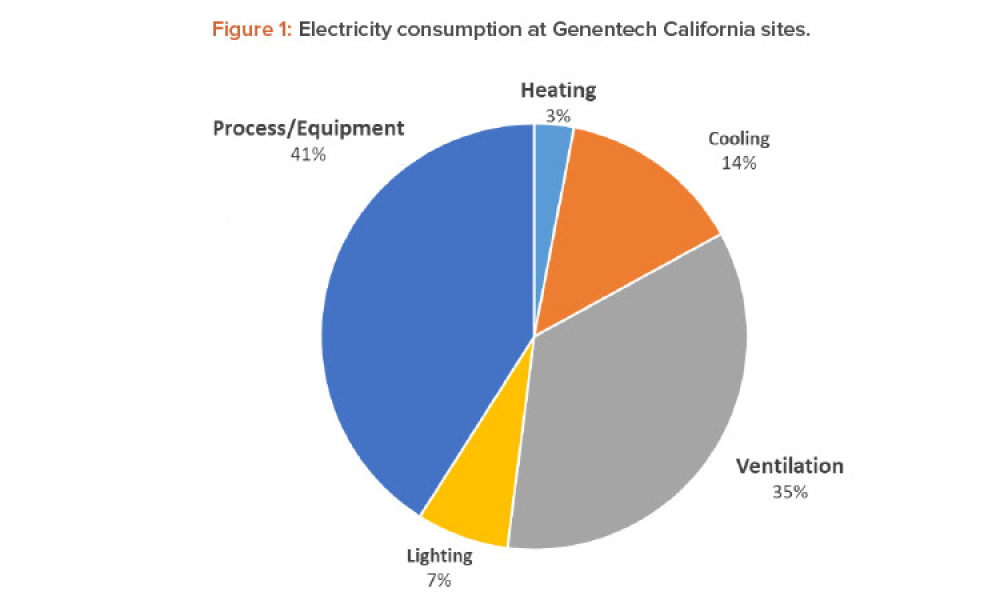
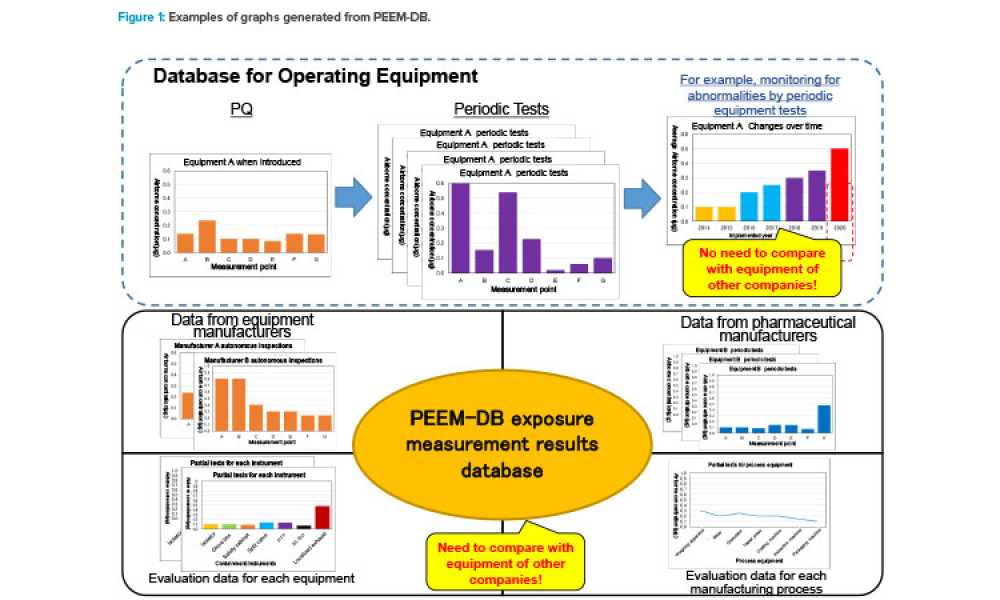
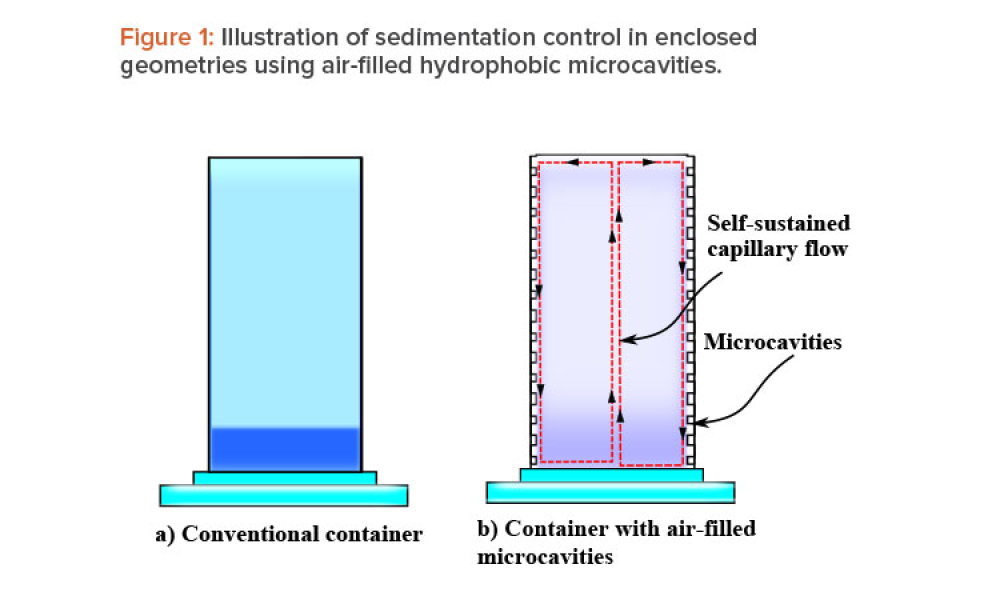
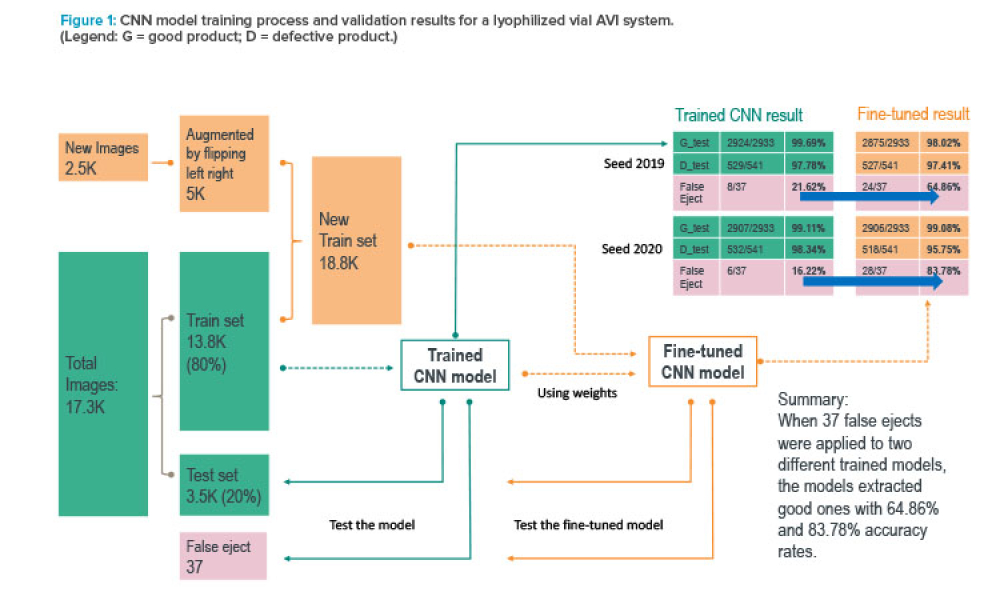
Containment (Containmt) is the action of confining within a defined space a microbiological agent or other entity that is being cultured, stored, manipulated, transported, or destroyed to prevent or limit its contact with people and the environment. Methods to achieve containment include physical and biological barriers and inactivation using physical or chemical means.1.Primary Containment. Addresses the protection of personnel and the immediate laboratory environment from exposure to infectious agents. It involves using closed containers or biological safety cabinets along with secure operating procedures.
Join the Conversation
As an ISPE member you can engage with 22 active CoPs, including new communities focused on Artificial Intelligence and Sustainability. Connect with experts and join the conversations: Become an ISPE member
ISPE members: Get more involved by volunteering.
Guidance Documents
Biotechnology (1)
+Containment (4)
+Microbiological & Viral Contamination Control (3)
+Quality by Design (1)
+Sustainability (1)
+Sustainable Facilities, HVAC, & Controlled Environments (2)
+Community Discussions
Community Discussions
Pharmaceutical Engineering Magazine Articles
Featured Conferences

iSpeak Blog Posts
Conferences