Downloads
2020 ISPE Barrier Survey: Tracking the Journey of Barrier Technology
Cover: For over two decades, the ISPE Barrier Isolator Survey has gathered meaningful data on the applications of barrier technology and has been a resource for the fill-finish pharmaceutical industry community. This article provides context for the latest survey, the first in several years, and presents its key results, which were first shared at the 2020 ISPE Aseptic Conference.
Case Study: Facilitating Efficient Life-Cycle Management via ICH Q12
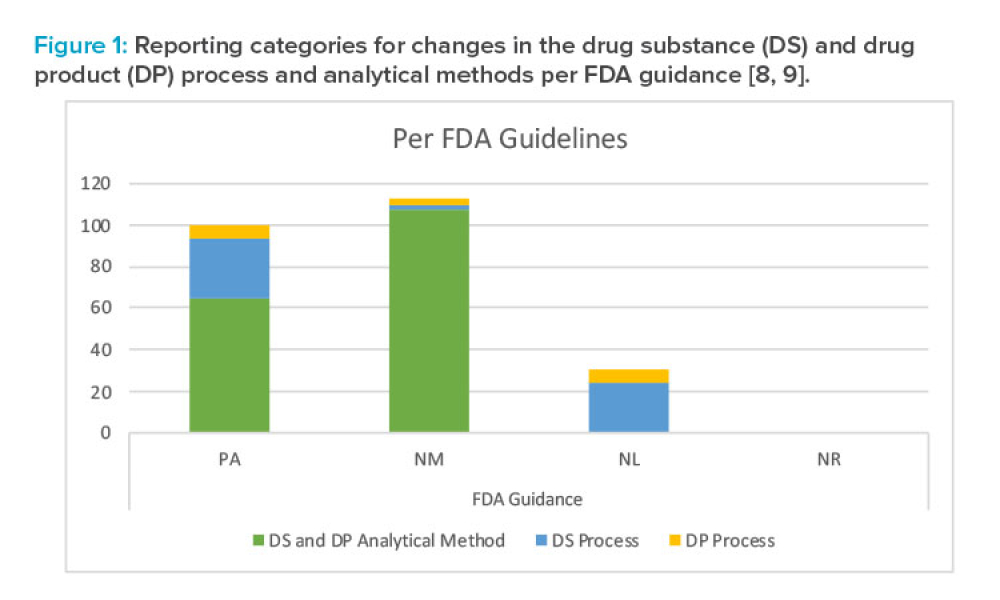
Feature: The latest ICH guideline, ICH Q12, introduces regulatory mechanisms, such as established conditions (ECs), to simplify and expedite postapproval product variations and enable continual product improvement. As illustrated by this case study for a small molecule product, the appropriate use of established conditions can successfully narrow the technical and regulatory gaps that limited the realization of flexible regulatory approaches promised by the application of Quality by Design principles.
Special Report: COVID-19 Impact
Special Report:
- Pharmaceutical Engineering® COVID-19 Impact Survey: How the Industry Is Responding
- Engage with Health Authorities to Mitigate & Prevent Drug Shortages
- Pandemic Preparedness & Business Continuity
- How Vaccines Are Developed
Process Technology
TECHNICAL: Lyophilizer Instrument Calibration: Principles and Practices Historically, the pharmaceutical industry’s focus has been on the lyophilization process and equipment, but discussion about calibration of process monitoring and control instrumentation has been quite limited. A greater understanding of the science and technology of lyophilization drives improvements in calibration, which leads to better process control and increased confidence in achieving product quality.
In This Issue
Historically, the pharmaceutical industry’s focus has been on the lyophilization process and equipment, but discussion about calibration of process monitoring and control instrumentation has been quite limited. Recently, focused attention has been given to control and monitoring instrumentation for lyophilization.
Continued process verification (CPV) as defined in the US FDA process validation guideline
ISPE has a new special interest group (SIG) to work on IT cybersecurity. The Special Interest Group was formed under GAMP®. A conversation with Jason Young of Silver Bullet Security, who heads the new group, provides details about the Special Interest Group.
Between 2009 and 2019, the number of adverse events (AEs) for drugs and therapeutic biologic products recorded by the US FDA Adverse Event Reporting System (FAERS) increased more than 300%, from 490,032 to 2.19 million cases (as of 31 December 2019).
Vaccine development is an intricate undertaking, which may involve numerous challenges from the initial process of identifying an antigen to the final steps of delivering and administering the licensed product. The COVID-19 pandemic has put a spotlight on the science of vaccine development. As the world awaits a vaccine for the coronavirus, manufacturers face unprecedented pressure to respond...
Recent projects on serialization and track and trace help illustrate the concepts of vertical and horizontal integration. With vertical integration, the unique product identification information (serial number, lot, etc.) used by sensors and printers on the packaging lines is made accessible to the supply chain and regulatory hubs throughout the entire technology stack. With horizontal...
This article updates a 2006 Pharmaceutical Engineering® Online Exclusive article titled “Avian Flu—Is My Company Prepared?” by Wendy Haines and Martin Rock.
When faced with large-scale disruptive events such as the COVID-19 pandemic, the emergency and business continuity plans of drug manufacturers and event-specific actions of health authorities may not always be sufficient to prevent shortages of critical medical products. However, early, transparent engagement with health authorities is a powerful opportunity for drug manufacturers to...
The 2020 ISPE Aseptic Conference was capped off on Tuesday, 3 March, by the Interactive Regulatory Panel session, which has been a popular feature of the conference during its 29 years. The session featured a panel...
Since January, nothing has been normal. Nothing, especially when we try to compare it to anything we have experienced in the recent past. So how do we begin to move forward as the efforts of both the public and private sectors combine to offset the advance of the impact to businesses due to COVID-19?
I wanted to take a break from my usual column and highlight a few Young Professionals (YPs) around the world. These individuals have not let COVID-19, or anything else, get in the way of their career development. Each joined...
How is your calendar looking these days? As I write this, we have all been living a bit differently today than we were one year ago. I’ll bet you have seen an increase in webinars, virtual trainings, and video conference calls, and likely having far fewer face-to-face interactions.
For over two decades, the ISPE Barrier Isolator Survey has gathered meaningful data on the applications of barrier technology and been a resource for the fill-finish pharmaceutical industry community. This article provides context for the latest survey, the first in several years, and presents its key results, which were first shared at the