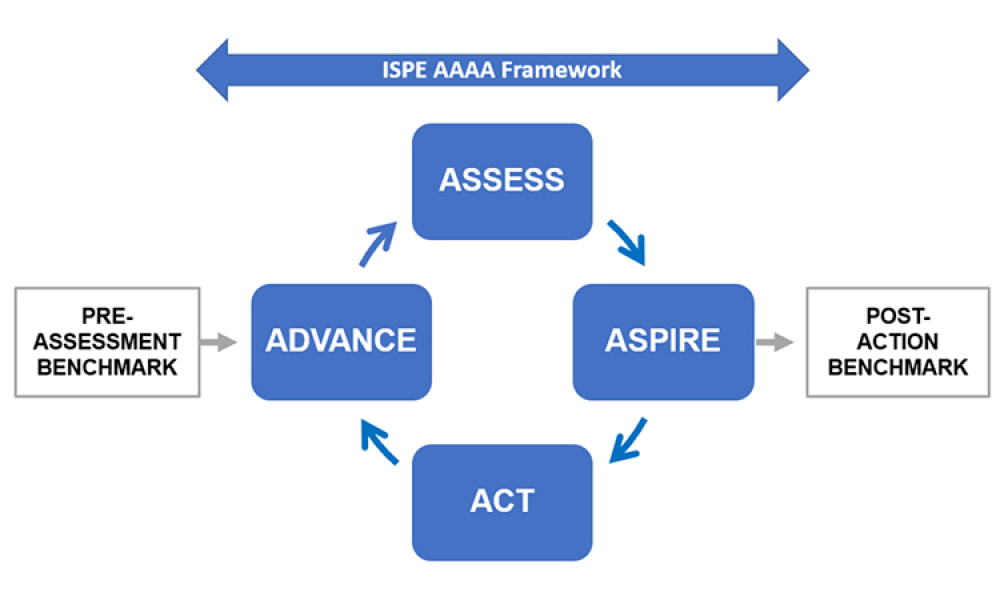
The needs of patients must always be at the forefront of what we do.
Many of our colleagues are taking on the challenge of rethinking the old ways. They are collaborating with each other and the regulatory agencies to take effective steps to remain at the forefront of regulatory science and innovation. Why? In order to ensure the continued delivery of effective medicines.