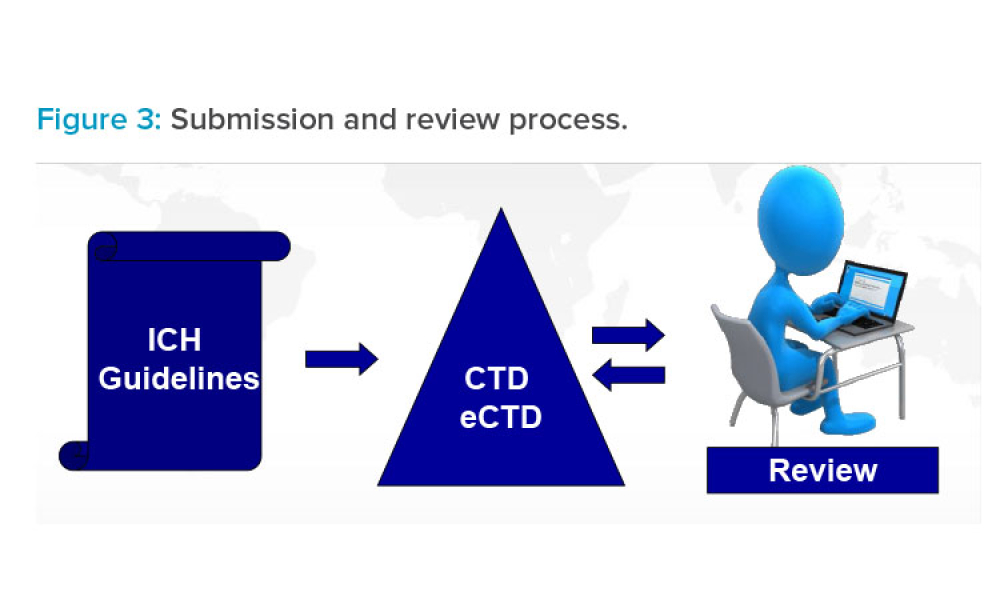
In its 30-year history, the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) has covered a wide range of topics to generate quality, safety, efficacy, and multidisciplinary harmonized guidelines. As science advances, issued guidelines are being updated and new guidelines are proposed in a pipeline of potential future activity. This...

Downloads
A Vision for ICH Q12: Current Experience, Future Perspectives
Cover: Management of global postapproval chemistry, manufacturing, and controls changes is a growing challenge for industry with many issues. ICH Q12 (Technical and Regulatory Considerations for Pharmaceutical Product Lifecycle Management) is a transformative document shaping global regulatory postapproval submissions that will help alleviate some of these issues.
ICH Quality Guidelines: Present Initiatives and ISPE Involvement
Feature: The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) has covered a wide range of topics to generate quality, safety, e cacy, and multidisciplinary harmonized guidelines. This article summarizes all quality and related multidisciplinary guidelines and discusses new topics and ISPE’s role in guideline development and implementation.
Distant Assessments, Audits, and Regulatory Guidance
Feature: The ISPE Global Pharmaceutical Regulatory Summit, held virtually on 28 April 2021, brought together 11 regulators from different parts of the world to discuss how their approaches to GMP inspections have adapted to the COVID-19 pandemic.
Pharmaceutical Cleanroom Design and ISO 14644-16
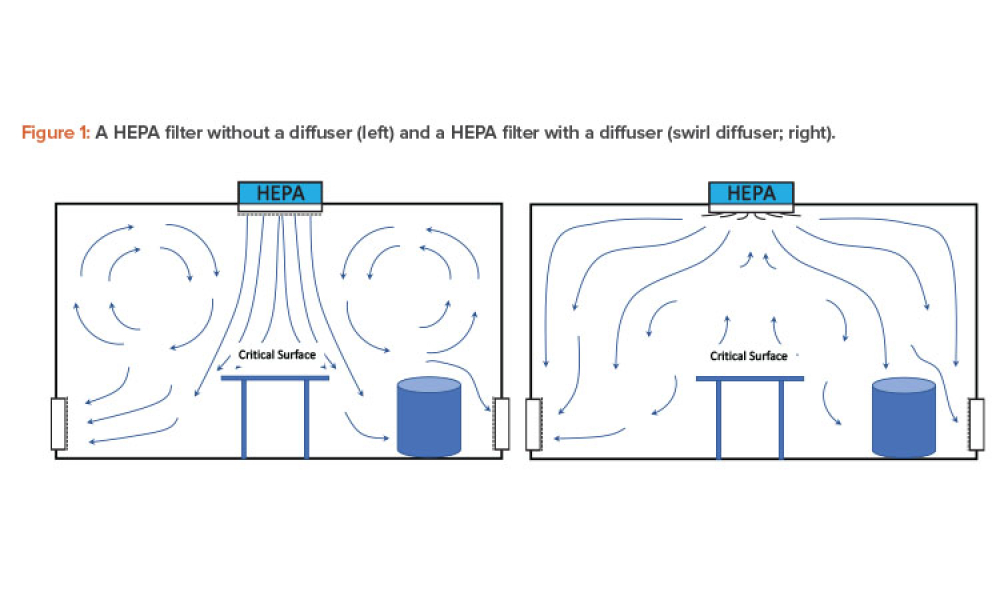
TECHNICAL: Cleanrooms and controlled contamination environments are increasingly being used across many industrial sectors, including the pharmaceutical industry. An important issue is the operating cost associated with cleanroom energy consumption and, consequently, the identification of applicable energy containment measures. This article reviews pharmaceutical cleanroom calculations for non-unidirectional airflow against energy consumption with known sources of contamination and type of air diffusion used. It proposes alternative cases to compare potential economic savings from applying energy-saving measures proposed by ISO 14644-16.
Temperature and Humidity Requirements in Pharmaceutical Facilities
TECHNICAL: Defining room temperature and humidity limits is a frequent topic of debate when designing and operating pharmaceutical and biotechnology facilities. What are appropriate alarm limits and acceptable durations for an alarm condition? Understanding the source of temperature and humidity requirements, and strategies for setting limits, can ensure both compliance and optimum use of energy. This article provides guidance on these topics, with supporting rationales.
In This Issue
The ISPE Global Pharmaceutical Regulatory Summit, held virtually on 28 April 2021, brought together 11 regulators from different parts of the world to discuss how their...
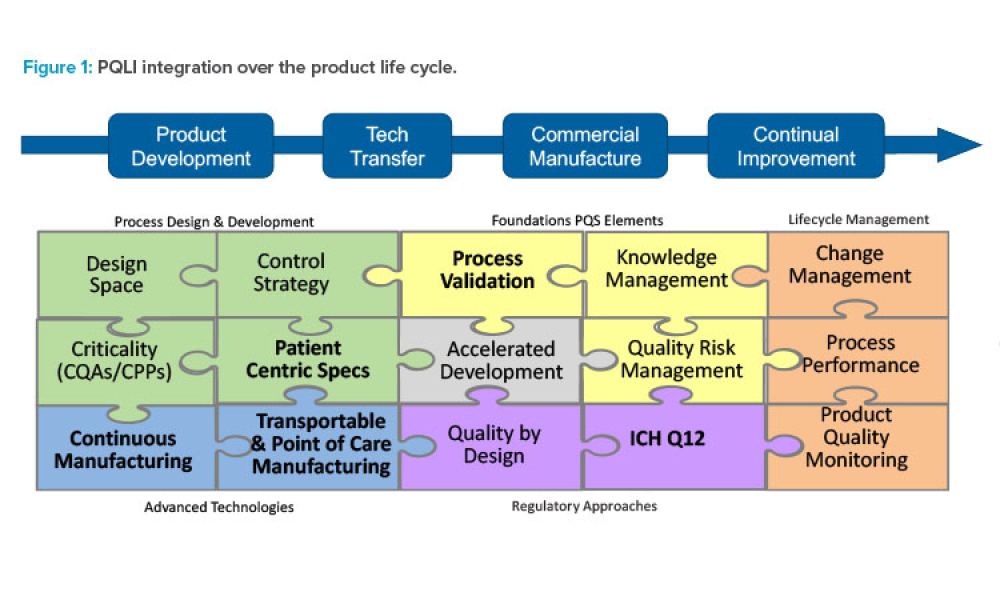
A unique aspect of the pharmaceutical industry is the pairing of innovation and regulation. For nearly two decades, ISPE’s Product Quality Lifecycle Initiative (PQLI®) has worked at the nexus of...
ISPE’s Regulatory Affairs function plays a vital role in the Society, which is to build effective relationships with regulators and agencies globally and ensure all members have access to the latest regulatory developments and...
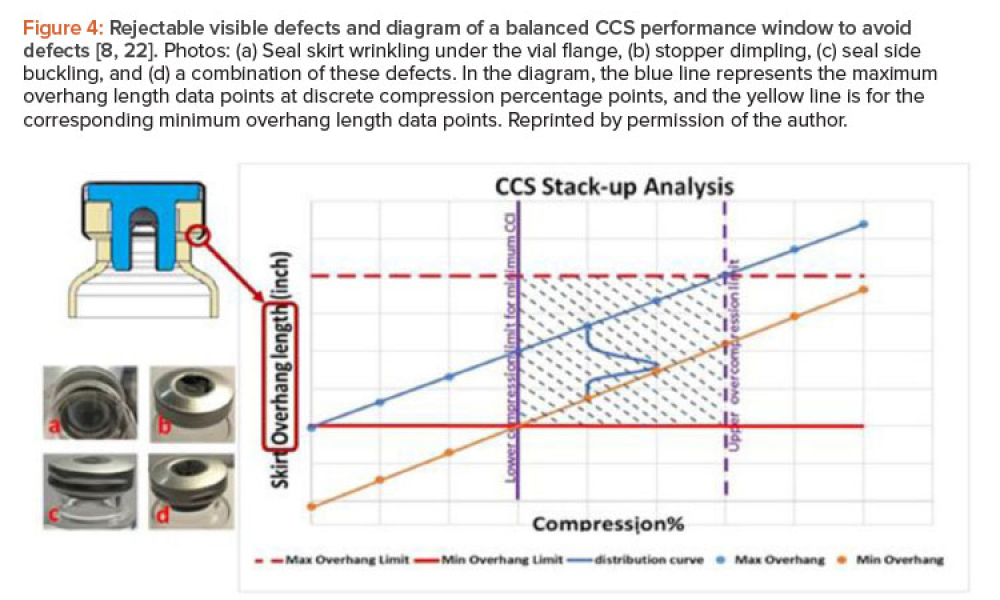
Any systematic pharmaceutical engineering approach for ensuring vial container closure system (CCS) performance must include choosing qualified container closure system components, the proper pharmaceutical process setup, and applicable testing methods. Container closure integrity (CCI) is an essential part of container closure system performance. A holistic strategy is needed to qualify...
Japan’s pharmaceutical market is one of the world’s largest and the ISPE Japan Affiliate is helping its members stay connected and current in the ever-changing pharmaceutical industry.
The second guide in ISPE’s Advancing Pharmaceutical Quality (APQ) series provides a systematic and proactive approach to quantitatively assessing and advancing leadership systems by evaluating the management responsibilities highlighted in ICH Q10 as well as other key leadership components.
Cleanrooms and controlled contamination environments are increasingly being used across many industrial sectors, including the pharmaceutical industry. An important issue is the operating cost associated with cleanroom energy consumption and, consequently, the identification of applicable energy containment measures. This article reviews pharmaceutical cleanroom calculations for...
Defining room temperature and humidity limits is a frequent topic of debate when designing and operating pharmaceutical and biotechnology facilities. What are appropriate alarm limits and acceptable durations for an alarm condition? Understanding the source of temperature and humidity requirements, and strategies for setting limits, can ensure both compliance and optimum use of energy. This...
I live in Indianapolis, Indiana, truly a great place to live. It is not difficult to understand why I like Indy so much: humble, down-to-earth people, farming (I grew up on a farm), readily available participant sports, and many spectator sporting event opportunities including football, soccer, baseball, and racing. Especially during the month of May, racing and the Indy 500 in particular are...
Excitement for restarting face-to-face conferences is building and as plans at local and international levels are in progress, we continue to enjoy the benefits of remote interactions. Innovation and collaboration achieved through fully virtual conferences and seminars in the past year and a half show us that both have great benefits.
Innovation is generally associated with driving business outcomes and processes, adapting to technology, and developing creative management approaches. In the pharmaceutical industry, innovation takes on a more extensive and specific meaning. It is acknowledged to be continuous as it involves dynamic thinking on how to integrate technology into drug development, manufacturing processes, and...
Management of global postapproval chemistry, manufacturing, and controls (CMC) changes is a growing challenge for industry with many issues. ICH Q12 (Technical and Regulatory Considerations for Pharmaceutical Product Lifecycle Management) is a transformative document shaping global regulatory postapproval submissions that will help alleviate some of these issues.






![Figure 1: Seasonal comfort (temperature and RH). ©ASHRAE, www.ashrae.org. Used with permission from 2017 ANSI/ASHRAE Standard 55 [4].](/sites/default/files/styles/teaser_image/public/2021-08/0921_PE_SO_TechHaycock_01.jpg?itok=mJiPGN_X)