Historically, cell therapies are used to treat patients with cancer after relapse from other approved treatment modalities, or if no approved treatment is available. However, the introduction of allogeneic cell therapies has created exciting opportunities to broaden access to cell-based treatments. With advancements in manufacturing, developers are becoming increasingly interested in...

Downloads
AI’s Promise for ATMPS
Cover: Industry 4.0 applications in biopharma involve the complete spectrum of data science throughout the entire product life cycle of many disparate entity types. Tools such as digitalization, modern data science, and the industrial internet of things are becoming requirements for modern implementations including advanced therapy medicinal products (ATMPs). This article addresses artificial intelligence and how it can help deliver on the promise of Industry 4.0 approaches to the entire product life cycle, from biology and medicine through product research and development, to validation, manufacturing, and post-launch surveillance.
Supporting Scalable Cell Therapy Using Allogeneic Workflows
Feature: Historically, cell therapies are used to treat patients with cancer after relapse from other approved treatment modalities, or if no approved treatment is available. However, the introduction of allogeneic cell therapies has created exciting opportunities to broaden access to cell-based treatments. With advancements in manufacturing, developers are becoming increasingly interested in commercializing allogenic therapies, bringing them one step closer to becoming broadly available to more patients.
Personalized Medicine: The Industry’s Future
Feature: With so many options for personalizing our lives, is the personalization of medicine far behind? With all the data available, how can the industry bring personalized medicine to patients? This article explores what is currently available and where the pharmaceutical industry can move forward to better serve patient needs.
Continuous Process: Implementing Low pH Viral Inactivation in a Continuous Process
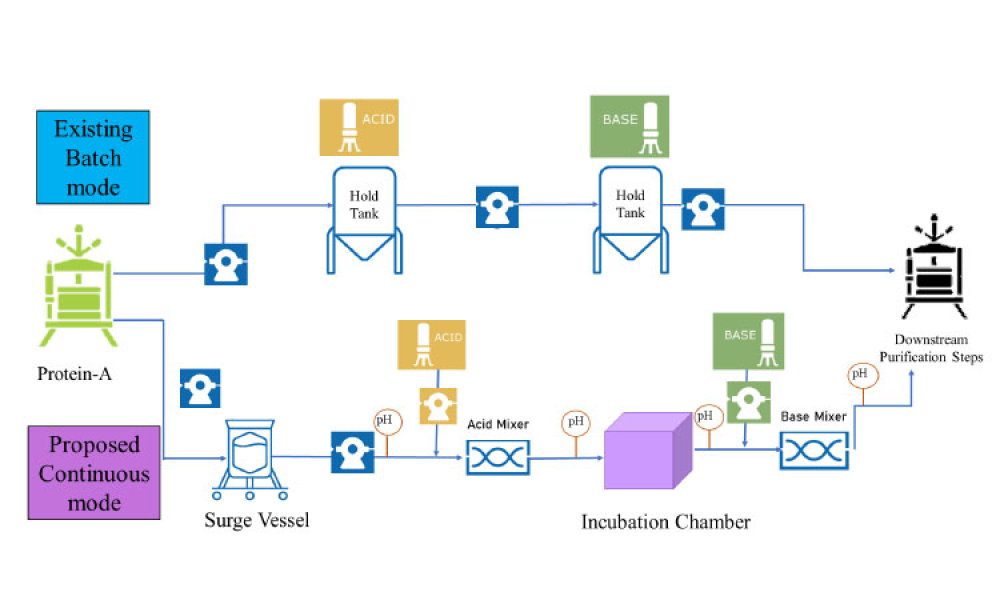
TECHNICAL: Viral inactivation (VI) is a critical step in ensuring the safety of monoclonal antibody (mAb), Fc fusion, and recombinant protein therapeutics and it is typically an important component of an overall virus control strategy for downstream biotherapeutic production processes. Considerations for successful implementation of an inline VI process are discussed in this article.
Software as a Service: The Journey to Becoming a Life Sciences SAAS Provider
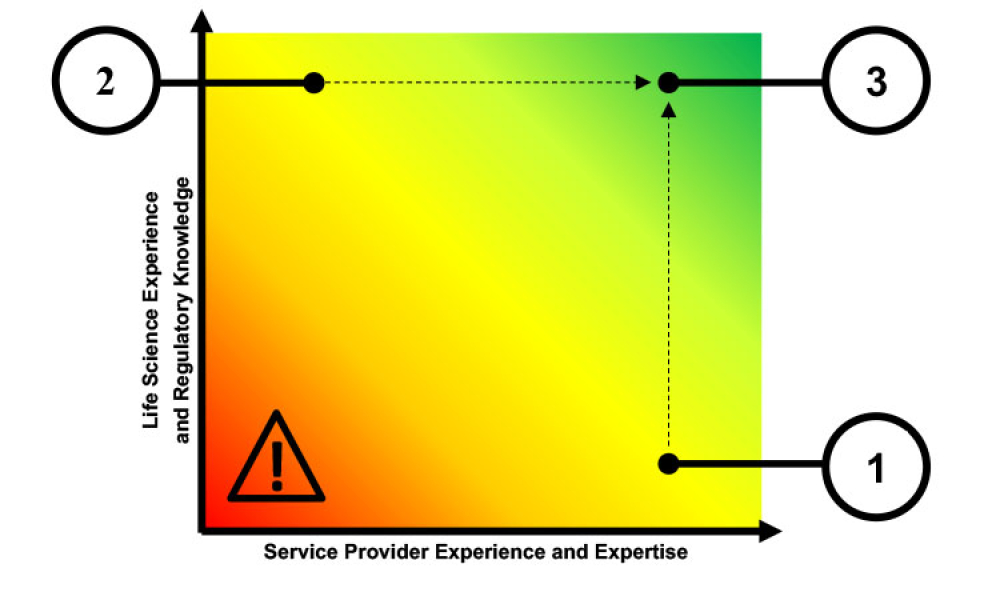
TECHNICAL: Use of software as a service (SaaS) applications within the life sciences industry is growing. This article reviews the issues and challenges faced by SaaS providers and identifi es the qualities of successful providers for this highly regulated industry.
In This Issue
With so many options for personalizing our lives, is the personalization of medicine far behind? With all the data available, how can the industry bring personalized medicine to patients? This article explores what is currently available and where the pharmaceutical industry can move forward to better serve patient needs.
The process of bringing new drugs and products to market requires creativity, thinking outside the box, and the courage to fail numerous times before making a single discovery. This rings especially true now, as the industry faces the COVID-19 pandemic and doubts about vaccines and therapies getting to market in record time to improve human health. This article presents a deep dive into gene...
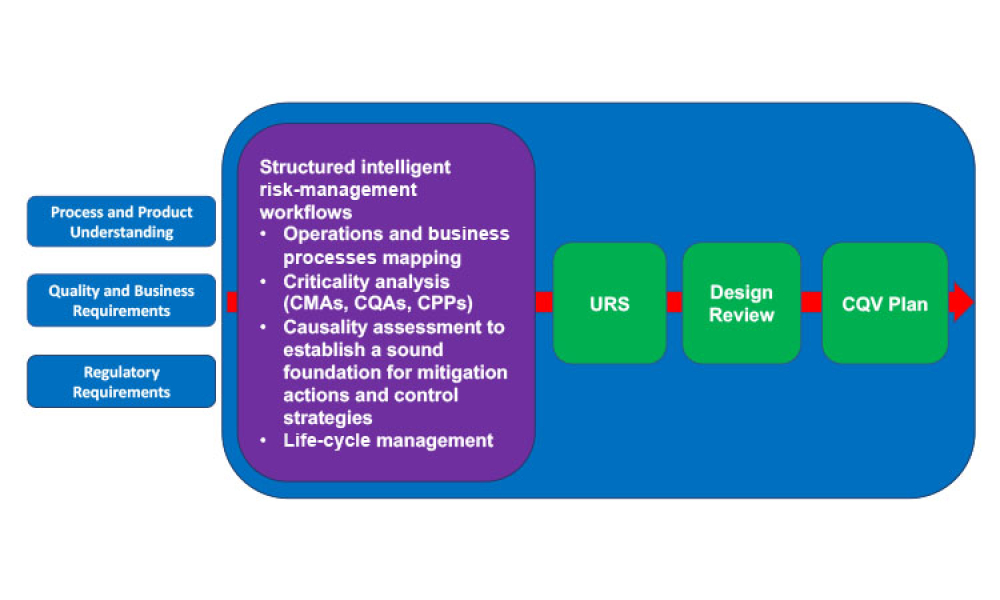
This article revisits the concept of phased engineering, procurement, and construction (EPC) and updates it with risk-based considerations specifically regarding the commissioning, qualification, and validation (CQV) of general life-cycle principles for pharma and biotech projects. Enhancing the relationship between phases of a project, advanced planning, and more formal management of...
The 2021 ISPE Biotechnology Conference and Workshop began on 22 September with a series of keynote presentations on a range of topics related to cell and gene therapies and...
“The only constant in life is change.” This quote is as true today as it was 2,500 years ago when Heraclitus laid out his philosophy. In the last five years, the word “cure” has been used to describe new therapies targeting many different cancer and hereditary diseases. The potential of these therapies to improve human health is profound. These breakthrough therapies utilize advancements in...
Viral inactivation (VI) is a critical step in ensuring the safety of monoclonal antibody (mAb), Fc fusion, and recombinant protein therapeutics and it is typically an important component of an overall virus control strategy for downstream biotherapeutic production processes. Considerations for successful implementation of an inline VI process are discussed in this article.
Use of software as a service (SaaS) applications within the life sciences industry is growing. This article reviews the issues and challenges faced by SaaS providers and identifies the qualities of successful providers for this highly regulated industry.
Thank you! It has been the honor of my life to serve as the ISPE International Board of Directors Chair in 2020–2021. I could never have imagined COVID-19 would still be such a significant issue at the end of my term.
The 2021 ISPE Annual Meeting & Expo brings my year as ISPE International Chair of Emerging Leaders (EL) and Emerging Leaders representative on the
It is easy to forget to enjoy your success. We all get so focused on the drive to be better, do more, prove ourselves, prove something, and grow that sometimes we forget to stop, be grateful, and enjoy and celebrate all of our success.
Industry 4.0 applications in biopharma involve the complete spectrum of data science throughout the entire product life cycle of many disparate entity types. Tools such as digitalization, modern data science, and the industrial internet of things (IIoT) exist now, and examples from other industries such as Siri and Alexa, face identification, and self-driving cars can guide their...